Deck 14: Gas Chromatography
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 14: Gas Chromatography
1
Why are the following procedures invalid?
(a) To minimize the time required for an analysis, the temperature of a GC instrument is increased above the boiling points of the components in the mixture.
(b) To increase the retention times and thus maximize the separation between peaks, the gas flow is adjusted to a very low value.
(a) To minimize the time required for an analysis, the temperature of a GC instrument is increased above the boiling points of the components in the mixture.
(b) To increase the retention times and thus maximize the separation between peaks, the gas flow is adjusted to a very low value.
(a) The gaseous compounds will not reach a liquid-vapor equilibrium with the liquid adsor- bent on the inert packing of the column.
(b) The peaks would becomevery broad by diffusion so tthat no advantage is gained.
(b) The peaks would becomevery broad by diffusion so tthat no advantage is gained.
2
Alcohols have very long retention times on Carbowax columns. Why? (Hint: Consider the polarity of alcohols.)
Alcohols can form hydrogen bounds with the oxygen atoms in a Carbowax.
3
Suppose an organic compound has a higher heat conductivity than the carrier gas. How would its GC signal appear on a chromatogram run on an instrument with
(a) a thermal conductivity detector?
(b) a flame ionization detector?
(a) a thermal conductivity detector?
(b) a flame ionization detector?
(a) The signal will appear as a dip from the baseline instead of a peak rising from the baseline because the sample arm becomes cooler (instead of hotter) relative to the feference arm in the Wheatstone bridge.
(b) The signal will appear as a normal peak because a flame ionizaiton detector is not affected by thermal conductivity.
(b) The signal will appear as a normal peak because a flame ionizaiton detector is not affected by thermal conductivity.
4
The RT for cyclohexane on a GC instrument is 0.75 min. The temperature of the GC is 70?C and the gas flow rate is 60 mL / min.
(a) Would the RT be higher or lower if the column temperature is raised to 100?C?
(b) Would the RT be higher or lower if the flow rate is changed to 30 mL / min?
(a) Would the RT be higher or lower if the column temperature is raised to 100?C?
(b) Would the RT be higher or lower if the flow rate is changed to 30 mL / min?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Between sample injections, some students clean a microsyringe with acetone rather than with the mixture to be analyzed. Why is this a poor technique?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Helium is the carrier gas of choice for a gas chromatograph containing a thermal conductivity detector, and nitrogen gas is preferred for a gas chromatograph having a flame ionization detector. Suggest a reason for this.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
Suppose that, on a GC column, methyl hexanoate eluted in 1.87 minutes, methyl heptanoate eluted in 2.35 minutes, and methyl octanoate eluted in 3.0 minutes. What is the structure of an unknown methyl ester, known to belong to the same homologous series, that elutes at 4.7 min- utes?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
A student at another university wants to duplicate a GC separation that you did in lab. What information about the instrument and column conditions should you send him?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the following compounds:
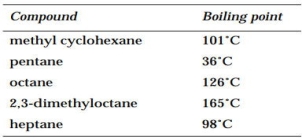 If a mixture of these compounds is run on a GC using an OV-101 column, which compound will have the lowest RT? the highest?
If a mixture of these compounds is run on a GC using an OV-101 column, which compound will have the lowest RT? the highest?
 If a mixture of these compounds is run on a GC using an OV-101 column, which compound will have the lowest RT? the highest?
If a mixture of these compounds is run on a GC using an OV-101 column, which compound will have the lowest RT? the highest?
Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the following compounds:
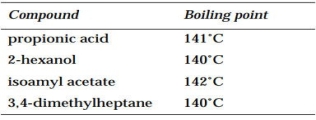 If a mixture of these compounds is run on a GC using a DEGS column, which compound will have the lowest RT? the highest?
If a mixture of these compounds is run on a GC using a DEGS column, which compound will have the lowest RT? the highest?
 If a mixture of these compounds is run on a GC using a DEGS column, which compound will have the lowest RT? the highest?
If a mixture of these compounds is run on a GC using a DEGS column, which compound will have the lowest RT? the highest?
Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


