Deck 16: Infrared Spectroscopy
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/6
Play
Full screen (f)
Deck 16: Infrared Spectroscopy
1
Arrange the following list of compounds in order of increasing intensity of the C=C stretching absorption (least-intense first), assuming identical molar concentrations in the sample cell:
(a) CH3CH=CHCH2CH3
(b) CH2=CHCH2CH3
(c) CH2=CCl2
(a) CH3CH=CHCH2CH3
(b) CH2=CHCH2CH3
(c) CH2=CCl2
a < b < c, based on increasing bond moment of C=C.
2
The infrared spectrum of a student's sample shows a weak absorption band at 3710 cm-1, yet the student is positive that the compound is not an alcohol or amine. Explain.
If the student's sample is a carbonyl compound, the absorption probably arises from a carbo- nyl vibrational overtone. The absorption could also arise from dirty NaCl plates.
3
A double peak around 3300 cm-1 always indicates the presence of -NH2.
Explain your answer.
Explain your answer.
False
4
A student runs the infrared spectrum of cyclopentanone using chloroform as the solvent. The infrared spectrum shows a double carbonyl peak. Explain.

Unlock Deck
Unlock for access to all 6 flashcards in this deck.
Unlock Deck
k this deck
5
The infrared spectrum (thin-film) of a compound with the molecular formula C7H5N shows weak absorption at 3100 cm-1, moderately strong absorption at 2230 cm-1, and three peaks between 1400-1500 cm-1 as the principal absorption. Suggest a structure for this compound.

Unlock Deck
Unlock for access to all 6 flashcards in this deck.
Unlock Deck
k this deck
6
Tell how you would distinguish between each of the following pairs of compounds by their infrared spectra alone.
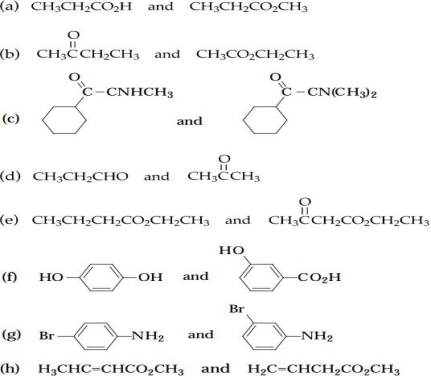


Unlock Deck
Unlock for access to all 6 flashcards in this deck.
Unlock Deck
k this deck


