Deck 6: Energy and Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/17
Play
Full screen (f)
Deck 6: Energy and Metabolism
1
Why do we have storage macromolecules, such as fats, in our bodies?
A) Macromolecules, such as fats, are a convenient way to store kinetic energy.
B) Human cells can directly capture the energy of sunlight through photosynthesis and store it as macromolecules such as fats.
C) Breaking down macromolecules, such as fats, is an endergonic process.
D) We can break down these macromolecules to provide energy for the endergonic reactions in our bodies.
A) Macromolecules, such as fats, are a convenient way to store kinetic energy.
B) Human cells can directly capture the energy of sunlight through photosynthesis and store it as macromolecules such as fats.
C) Breaking down macromolecules, such as fats, is an endergonic process.
D) We can break down these macromolecules to provide energy for the endergonic reactions in our bodies.
We can break down these macromolecules to provide energy for the endergonic reactions in our bodies.
2
It is summer, and you are excited about going to your local amusement park, and specifically about riding the new roller coaster that was just built. You imagine waiting at the top of the stairs for the roller coaster to pull into the station, climbing into the car, strapping yourself into the seatbelt, and pulling down the harness. You can imagine the cars slowly chugging up to the top of the first hill, coming down on the other side, and the excitement you expect to feel as you go along for the ride. Of all of the things that you have imagined, which is an example of potential energy?
A) Climbing into the car
B) Waiting at the top of the stairs for the roller coaster to pull into the station
C) The roller coaster car going up the first hill
D) Pulling down the harness
A) Climbing into the car
B) Waiting at the top of the stairs for the roller coaster to pull into the station
C) The roller coaster car going up the first hill
D) Pulling down the harness
Waiting at the top of the stairs for the roller coaster to pull into the station
3
In an experiment described in a chemistry lab book, the directions state that after mixing two chemicals (A and B) and waiting 5 minutes that A will be oxidized. This means that
A) chemical A has gained energy in the form of heat from chemical B.
B) chemical A has gained electrons from chemical B.
C) chemical A has reacted with oxygen.
D) chemical A has lost energy in the form of heat to chemical B.
E) chemical A has lost electrons to chemical B.
A) chemical A has gained energy in the form of heat from chemical B.
B) chemical A has gained electrons from chemical B.
C) chemical A has reacted with oxygen.
D) chemical A has lost energy in the form of heat to chemical B.
E) chemical A has lost electrons to chemical B.
chemical A has lost electrons to chemical B.
4
You eat a bowl of beans as part of your dinner. As you digest the beans, the proteins that are present get broken down to their component amino acids. As your body destroys the macromolecules that were present in the beans, is the energy present in those molecules destroyed?
A) No. The energy contained within these macromolecules is converted into other forms of chemical energy and kinetic energy, though some is lost as heat.
B) Yes. By breaking down these macromolecules, some of the energy they contained is destroyed.
C) No. Breaking down molecules does not lead to the release of energy.
D) No. While the vast majority of the energy contained in these macromolecules is converted to heat, it is not actually destroyed.
A) No. The energy contained within these macromolecules is converted into other forms of chemical energy and kinetic energy, though some is lost as heat.
B) Yes. By breaking down these macromolecules, some of the energy they contained is destroyed.
C) No. Breaking down molecules does not lead to the release of energy.
D) No. While the vast majority of the energy contained in these macromolecules is converted to heat, it is not actually destroyed.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
5
Glucose is broken down through cellular respiration, which involves a large number of chemical reactions. At the end of the cellular respiration process, a large number of ATP molecules are generated, yet not all of the possible energy that is contained in a molecule of glucose can be harnessed through these chemical reactions to generate ATP. In other words, during cellular respiration, not all of the energy that is contained in a molecule of glucose is converted into the energy stored in ATP. The remaining energy is
A) lost as heat.
B) donated to molecules in the cellular respiration process to reduce them.
C) destroyed.
D) used to drive exergonic reactions.
A) lost as heat.
B) donated to molecules in the cellular respiration process to reduce them.
C) destroyed.
D) used to drive exergonic reactions.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
6
Many metabolic pathways are ultimately concerned with ATP; either with the generation of ATP, or with the requirement of ATP for that pathway to function. Why is ATP so important to metabolism?
A) ATP is a protein that serves as the energy currency of cells.
B) The phosphate groups of ATP are held together by unstable bonds that can be broken to release energy.
C) Hydrolysis of the bond between adenine and ribose in ATP is commonly used to release energy that can be used to drive other cellular reactions.
D) Hydrolysis of ATP is used to drive exergonic reactions.
A) ATP is a protein that serves as the energy currency of cells.
B) The phosphate groups of ATP are held together by unstable bonds that can be broken to release energy.
C) Hydrolysis of the bond between adenine and ribose in ATP is commonly used to release energy that can be used to drive other cellular reactions.
D) Hydrolysis of ATP is used to drive exergonic reactions.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
7
AMP-activated protein kinase (AMPK) is an enzyme that is activated by high levels of AMP in cells. If levels of AMP are high in cells, that means that levels of ATP are low. Once active, AMPK activates catabolic pathways and inhibits anabolic pathways in the cell. Why do you think that is the case? Choose the answer that best explains the role of AMPK.
A) High levels of AMP indicate that there is a high amount of energy stored in the cell, thus activating catabolic pathways and inhibiting anabolic pathways are mechanisms to use stored energy.
B) By activating catabolic pathways, AMPK provides a mechanism to activate exergonic pathways, which is important if AMP levels are high in the cell.
C) Activating catabolic pathways and inhibiting anabolic pathways will ultimately lead to higher consumption of ATP, which is important if AMP levels are high in the cell.
D) By inhibiting anabolic pathways, AMPK provides a mechanism to generate heat for the cell, which is important if AMP levels are high in the cell.
A) High levels of AMP indicate that there is a high amount of energy stored in the cell, thus activating catabolic pathways and inhibiting anabolic pathways are mechanisms to use stored energy.
B) By activating catabolic pathways, AMPK provides a mechanism to activate exergonic pathways, which is important if AMP levels are high in the cell.
C) Activating catabolic pathways and inhibiting anabolic pathways will ultimately lead to higher consumption of ATP, which is important if AMP levels are high in the cell.
D) By inhibiting anabolic pathways, AMPK provides a mechanism to generate heat for the cell, which is important if AMP levels are high in the cell.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
8
Carbonic anhydrase (CA), an enzyme widely present in plant and animal tissues, catalyzes the reaction:
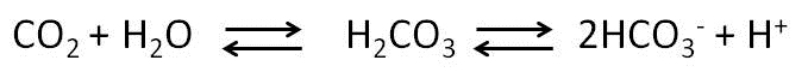 This reaction is important for the maintenance of acid base balance in blood and other tissues and to help transport CO2 out of tissues; without a catalyst, this reaction would not occur at rates that could maintain homeostasis. In the figure below, which line(s) represents the reaction catalyzed by carbonic anhydrase?
This reaction is important for the maintenance of acid base balance in blood and other tissues and to help transport CO2 out of tissues; without a catalyst, this reaction would not occur at rates that could maintain homeostasis. In the figure below, which line(s) represents the reaction catalyzed by carbonic anhydrase?
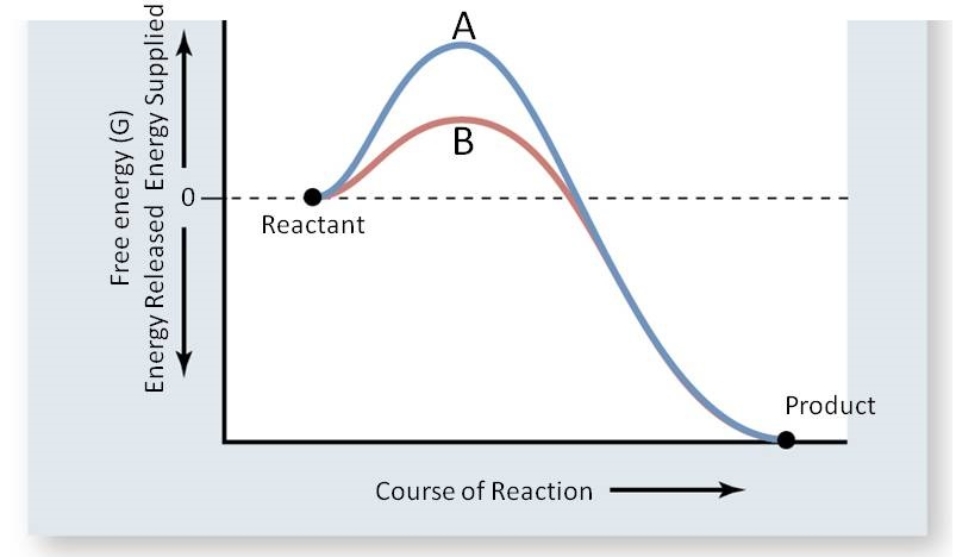
A) Line B; the activation energy has been lowered.
B) Line A; the activation energy increased.
C) Lines A and B; product is shown for both lines.
D) Line A; more energy is supplied.
E) Either, as the free energy is the same for both.
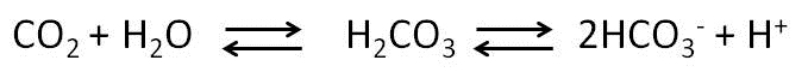 This reaction is important for the maintenance of acid base balance in blood and other tissues and to help transport CO2 out of tissues; without a catalyst, this reaction would not occur at rates that could maintain homeostasis. In the figure below, which line(s) represents the reaction catalyzed by carbonic anhydrase?
This reaction is important for the maintenance of acid base balance in blood and other tissues and to help transport CO2 out of tissues; without a catalyst, this reaction would not occur at rates that could maintain homeostasis. In the figure below, which line(s) represents the reaction catalyzed by carbonic anhydrase?
A) Line B; the activation energy has been lowered.
B) Line A; the activation energy increased.
C) Lines A and B; product is shown for both lines.
D) Line A; more energy is supplied.
E) Either, as the free energy is the same for both.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
9
Kinases are enzymes that can phosphorylate (transfer phosphate groups onto) macromolecules such as proteins. A particular kinase, Kinase 1 is known to promote cell division. It promotes cell division by phosphorylating Protein X. Phosphorylation of Protein X activates Protein X. Once activated, Protein X stimulates the production of other proteins such as Protein Y and Z that directly promote cell division. In order to function, Kinase 1 requires the presence of Metal A. However, in the presence of Protein A, Kinase 1 is nonfunctional. From the description, what is considered the substrate of Kinase 1?
A) Protein Y
B) Protein Z
C) Metal A
D) Protein X
E) Protein A
A) Protein Y
B) Protein Z
C) Metal A
D) Protein X
E) Protein A

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
10
Kinases are enzymes that can phosphorylate (transfer phosphate groups onto) macromolecules such as proteins. A particular kinase, Kinase 1 is known to promote cell division. It promotes cell division by phosphorylating Protein X. Phosphorylation of Protein X activates Protein X. Once activated, Protein X stimulates the production of other proteins such as Protein Y and Z that directly promote cell division. In order to function, Kinase 1 requires the presence of Metal A. However, in the presence of Protein A, Kinase 1 is nonfunctional. From the description, what is considered a cofactor of Kinase 1?
A) Protein A
B) Protein X
C) Protein Z
D) Protein Y
E) Metal A
A) Protein A
B) Protein X
C) Protein Z
D) Protein Y
E) Metal A

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
11
Based on the graph below, what are the optimal temperatures for the human enzyme and the hotsprings prokaryote enzyme? 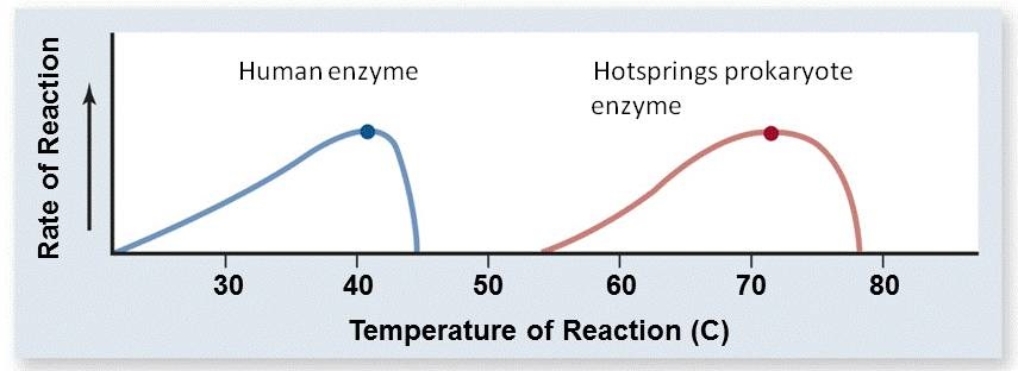
A) The optimal temperature for the human enzyme is 46oC; the optimal temperature for the hotsprings prokaryote enzyme is 79oC.
B) The optimal temperature for the human enzyme is 30oC; the optimal temperature for the hotsprings prokaryote enzyme is 60oC.
C) The optimal temperature for the human enzyme is 35oC; the optimal temperature for the hotsprings prokaryote enzyme is 65oC.
D) The optimal temperature for the human enzyme is 40oC; the optimal temperature for the hotsprings prokaryote enzyme is 72oC.

A) The optimal temperature for the human enzyme is 46oC; the optimal temperature for the hotsprings prokaryote enzyme is 79oC.
B) The optimal temperature for the human enzyme is 30oC; the optimal temperature for the hotsprings prokaryote enzyme is 60oC.
C) The optimal temperature for the human enzyme is 35oC; the optimal temperature for the hotsprings prokaryote enzyme is 65oC.
D) The optimal temperature for the human enzyme is 40oC; the optimal temperature for the hotsprings prokaryote enzyme is 72oC.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
12
The normal body temperature of a bat is similar to the normal body temperature of a human. Which of the curves below most likely represents an enzyme from a bat? 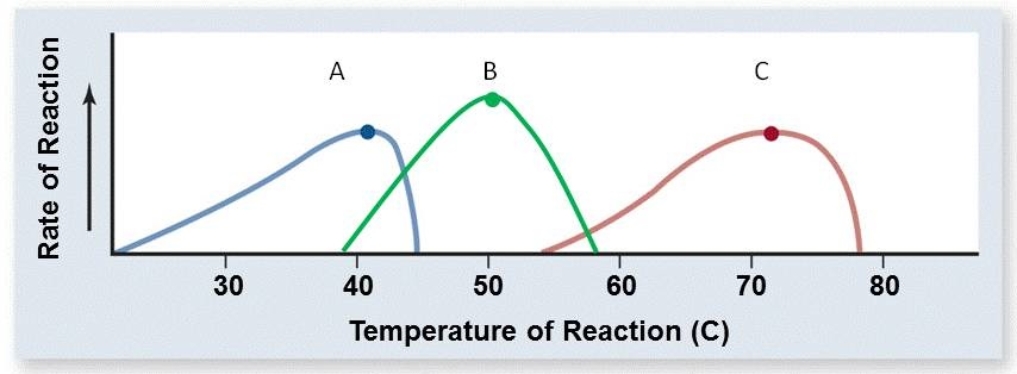
A) C
B) A
C) B

A) C
B) A
C) B

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
13
A current problem in modern medicine is the development of drug resistance mutations. This occurs when a mutation arises in a patient making him/her resistant to a drug and thus rendering the drug useless in treating a specific disease. Many useful drugs are competitive inhibitors of specific enzymes, and the drug-resistance mutations prevent the binding of the drug. These types of mutations, in addition to preventing competitive inhibitor binding, can also sometimes reduce the activity of the enzyme. Why is that the case?
A) These mutations most likely affect an allosteric site on the enzyme
B) These mutations lower the activation energy of the reaction catalyzed by the enzyme
C) Binding to the competitive inhibitor is essential for the function of the enzyme
D) These mutations most likely change the shape of the active site of the enzyme
A) These mutations most likely affect an allosteric site on the enzyme
B) These mutations lower the activation energy of the reaction catalyzed by the enzyme
C) Binding to the competitive inhibitor is essential for the function of the enzyme
D) These mutations most likely change the shape of the active site of the enzyme

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
14
Tacrolimus (FK-506) is a drug that inhibits an enzyme called calcineurin. Calcineurin is a protein phosphatase. This is an enzyme that dephosphorylates (removes phosphate groups from) proteins. When added to cells, tacrolimus can inhibit the dephosphorylation of a protein called NFAT, but it cannot prevent the dephosphorylation of a protein called CDK1. What is the most likely explanation for this finding?
A) NFAT is a substrate of calcineurin, but CDK1 is not.
B) Tacrolimus changes the optimum pH for calcineurin.
C) Tacrolimus is a competitive inhibitor of calcineurin for NFAT, but not for CDK1.
D) Calcineurin requires an additional cofactor to dephosphorylate NFAT.
A) NFAT is a substrate of calcineurin, but CDK1 is not.
B) Tacrolimus changes the optimum pH for calcineurin.
C) Tacrolimus is a competitive inhibitor of calcineurin for NFAT, but not for CDK1.
D) Calcineurin requires an additional cofactor to dephosphorylate NFAT.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
15
You are working with a specific enzyme-catalyzed reaction in the lab. You are a very careful experimentalist, and as a result, at the beginning of each of your experiments, you measure the temperature in the lab. On days 1 through 5, the temperature in the lab was 20oC. Today is day 6 of your experiment, and the temperature in the lab is 30oC. How do you predict that this will alter the rate of your enzyme-catalyzed reaction?
A) It could possibly increase or decrease the rate.
B) it will not affect the rate.
C) It will increase the rate.
D) It will decrease the rate.
A) It could possibly increase or decrease the rate.
B) it will not affect the rate.
C) It will increase the rate.
D) It will decrease the rate.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
16
A new antibiotic has been developed that inhibits the activity of an enzyme by competitive inhibition. What effect will this have on the activation energy of the enzyme-catalyzed reaction?
A) The activation energy required for the reaction in the presence of the antibiotic would be less than the activation energy required in the absence of the antibiotic.
B) The activation energy required for the reaction in the presence of the antibiotic will be the same as the activation energy required in the absence of the antibiotic.
C) The activation energy required for the reaction in the presence of the antibiotic would be greater than the activation energy required in the absence of the antibiotic.
A) The activation energy required for the reaction in the presence of the antibiotic would be less than the activation energy required in the absence of the antibiotic.
B) The activation energy required for the reaction in the presence of the antibiotic will be the same as the activation energy required in the absence of the antibiotic.
C) The activation energy required for the reaction in the presence of the antibiotic would be greater than the activation energy required in the absence of the antibiotic.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
17
Phosphofructokinase (PFK) is an enzyme that converts fructose 6-phosphate to fructose 1,6-bisphosphate, by adding a phosphate group. This is the first committed step of the metabolic pathway of glycolysis, and thus it is very tightly regulated. AMP binds to PFK at a site distinct from the binding site for fructose 6-phosphate, and stimulates the formation of fructose 1,6-bisphosphate. ATP binds to PFK at a site distinct from the binding site for fructose 6-phosphate, and inhibits the formation of fructose 1,6-bisphosphate. There are other regulators of this enzyme as well. What is the role of AMP in this example?
A) Competitive inhibitor
B) Noncompetitive inhibitor
C) Allosteric inhibitor
D) Allosteric activator
E) Catalyst
A) Competitive inhibitor
B) Noncompetitive inhibitor
C) Allosteric inhibitor
D) Allosteric activator
E) Catalyst

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck


