Deck 6: Addition Reactions of Alkenes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/13
Play
Full screen (f)
Deck 6: Addition Reactions of Alkenes
1
Which of the following alkenes is expected to have the highest heat of hydrogenation?
A) 1-pentene
B) trans-2-pentene
C) cis-2-pentene
D) 2-methyl-2-butene
A) 1-pentene
B) trans-2-pentene
C) cis-2-pentene
D) 2-methyl-2-butene
1-pentene
2
The product(s) in the following reaction is(are)
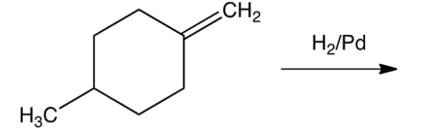
A) only trans-1-4-dimethylcyclohexane.
B) only cis-1-4-dimethylcyclohexane.
C) both trans and cis-1-4-dimethylcyclohexane.
D) methylcyclohexane.

A) only trans-1-4-dimethylcyclohexane.
B) only cis-1-4-dimethylcyclohexane.
C) both trans and cis-1-4-dimethylcyclohexane.
D) methylcyclohexane.
both trans and cis-1-4-dimethylcyclohexane.
3
What is the major product of the following reaction?
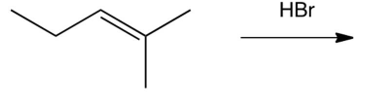
A)
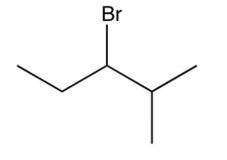
B)
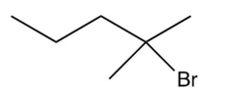
C)
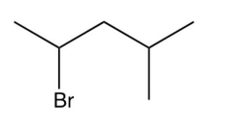
D)
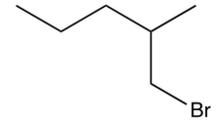

A)

B)

C)

D)


4
What is the intermediate in the following reaction?

A)
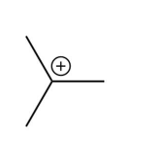
B)
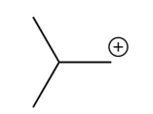
C)
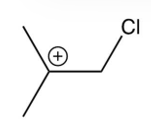
D)
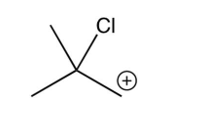

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
5
What is(are) the product(s) of the following hydroboration-oxidation reaction?
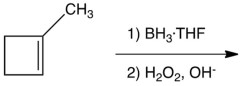
A) 1-methylcyclobutanol
B) trans-2-methylcyclobutanol
C) cis-2-methylcyclobutanol
D) equal amounts of 2 and 3

A) 1-methylcyclobutanol
B) trans-2-methylcyclobutanol
C) cis-2-methylcyclobutanol
D) equal amounts of 2 and 3

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following series of reactions would convert cyclohexanol to 1,2-epoxycyclohexane?
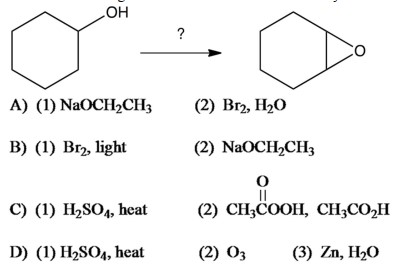
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
7
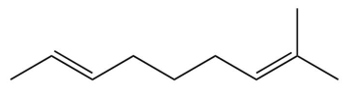
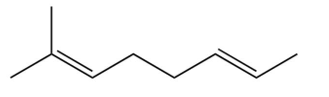
A compound is treated with ozone followed by zinc in water to give the following three products. Which structure below best fits the data?
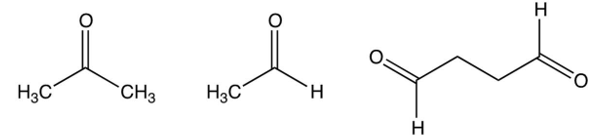
A)
B)
C)
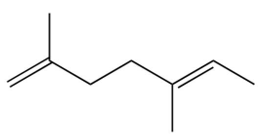
D)
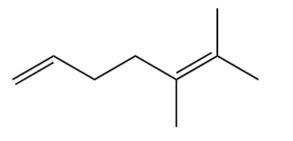

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following species is the intermediate in the bromination of propene?
A)
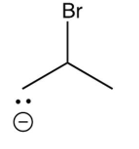
B)
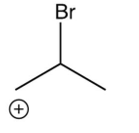
C)
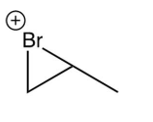
D)
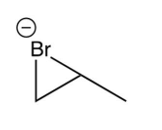
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
9
A compound, , is reacted with sodium ethoxide and gives a single elimination product, . Treatment with ozone followed by zinc and water gives the compound below. Identify the original compound.
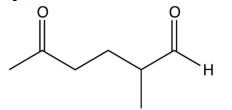
A) 2-chloro-1,1-dimethylcyclopentane
B) 1-chloro-1,2-dimethylcyclopentane
C) 4-chloro-1,2-dimethylcyclopentane
D) 2-chloro-1,3-dimethylcyclopentane

A) 2-chloro-1,1-dimethylcyclopentane
B) 1-chloro-1,2-dimethylcyclopentane
C) 4-chloro-1,2-dimethylcyclopentane
D) 2-chloro-1,3-dimethylcyclopentane

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
10
Which point on the potential energy diagram corresponds to the species below for the reaction of 2methylpropene with hydrogen chloride?
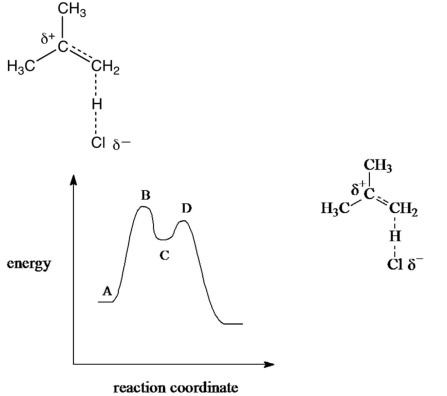
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the product of the reaction shown below?
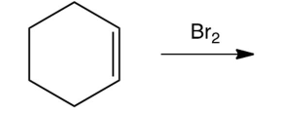
A)
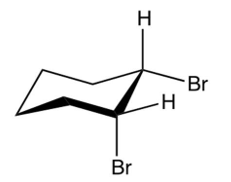
B)
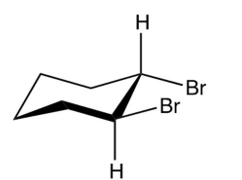
C)
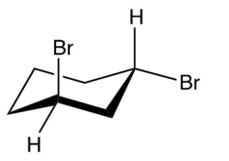
D)
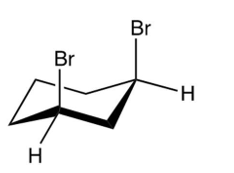

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
12
What product results from this reaction?
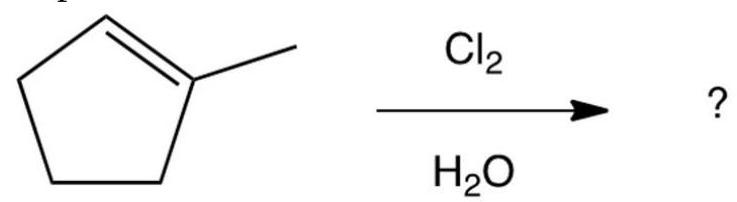
A)
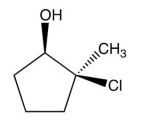
B)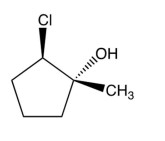
C)
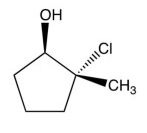
D)
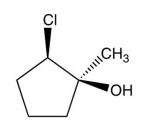

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck
13
The polymerization of styrene gives a polymer that has which structure?
A)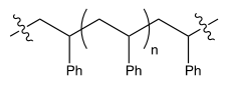
B)
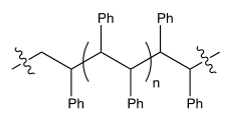
C)
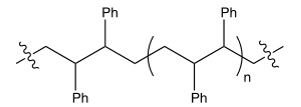
D) none of these

A)

B)

C)

D) none of these

Unlock Deck
Unlock for access to all 13 flashcards in this deck.
Unlock Deck
k this deck


