Deck 20: Enols and Enolates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/14
Play
Full screen (f)
Deck 20: Enols and Enolates
1
Which one of the following cannot form an enolate anion?
A) 2,2-dimethylbutanal
B) 2,3-dimethylbutanal
C) 2-ethylbutanal
D) 3,3-dimethylbutanal
A) 2,2-dimethylbutanal
B) 2,3-dimethylbutanal
C) 2-ethylbutanal
D) 3,3-dimethylbutanal
2,2-dimethylbutanal
2
Which one of the following has two different enol forms?
A) cyclohexanone
B) 3,3-dimethylcyclohexanone
C) 2,2-dimethylcyclohexanone
D) 4,4-dimethylcyclohexanone
A) cyclohexanone
B) 3,3-dimethylcyclohexanone
C) 2,2-dimethylcyclohexanone
D) 4,4-dimethylcyclohexanone
3,3-dimethylcyclohexanone
3
Identify the most acidic hydrogen in the following compound.
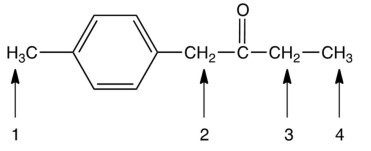
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4
2
4
What is the product of the reaction below?
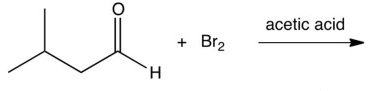
A)
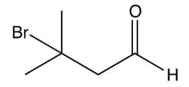
B)
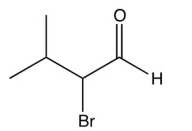
C)
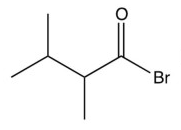
D)
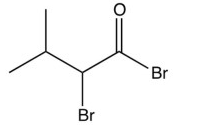

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following optically active compounds racemizes in dilute solution?
A)
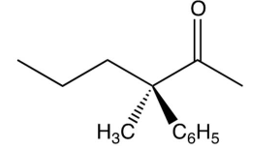
B)
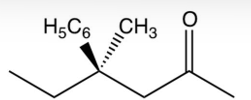
C)
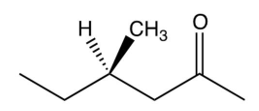
D)
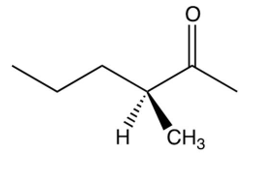
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
6
What is the aldol addition product of propanal?
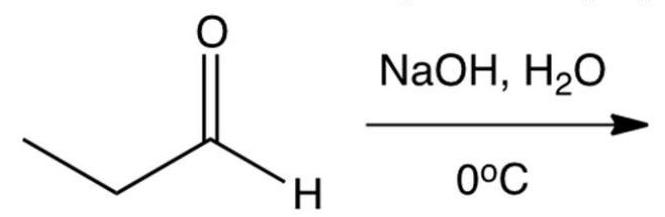
A) 2-hydroxy-2-methylpentanal
B) 3-hydroxy-2-methylpentanal
C) 3-hydroxyhexanal
D) 4-hydroxyhexanal

A) 2-hydroxy-2-methylpentanal
B) 3-hydroxy-2-methylpentanal
C) 3-hydroxyhexanal
D) 4-hydroxyhexanal

Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the starting reagent needed to make the following cyclic ketone by an intramolecular aldol condensation reaction.
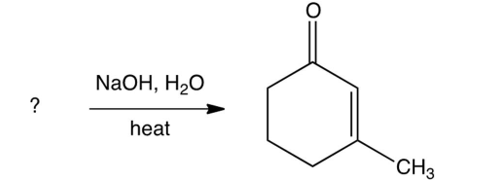
A)
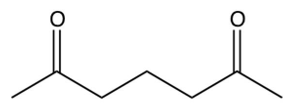
B)
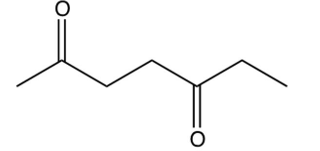
C)
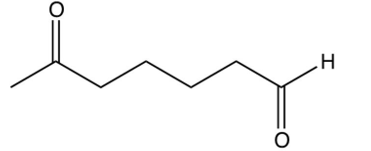
D)
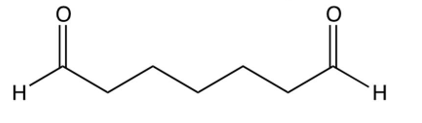

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
8
The Robinson annulation reaction is shown below. Identify the missing reagent in the first step.
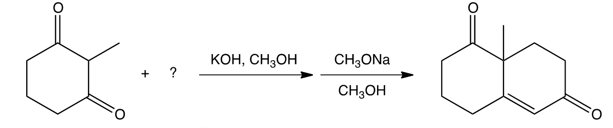
A)
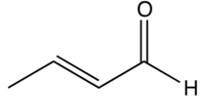
B)
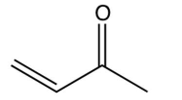
C)
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
9
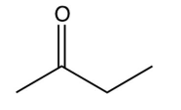
Which of the following is the Claisen condensation product of ethyl propanoate, ?
A)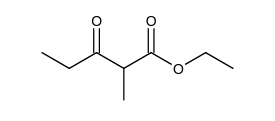
B)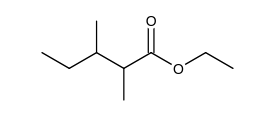
C)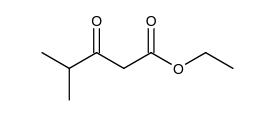
D)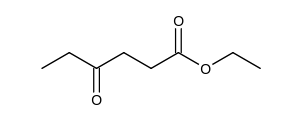
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the missing reagent in the reaction shown below.
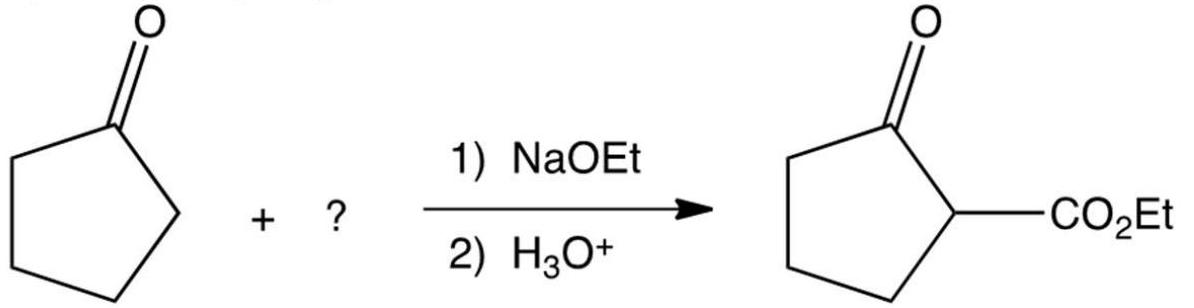
A) ethyl formate,
B) diethyl carbonate,
C) diethyl oxalate,
D) ethyl acetate,

A) ethyl formate,
B) diethyl carbonate,
C) diethyl oxalate,
D) ethyl acetate,

Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
11
What is the missing reagent in the synthesis shown below?
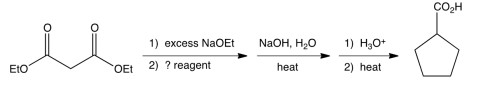
A) bromocyclopentane
B) 1,4-dibromobutane
C) 1,5-dibromopentane
D) 1,1-dibromocyclopentane

A) bromocyclopentane
B) 1,4-dibromobutane
C) 1,5-dibromopentane
D) 1,1-dibromocyclopentane

Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the Michael addition product of the reaction below?
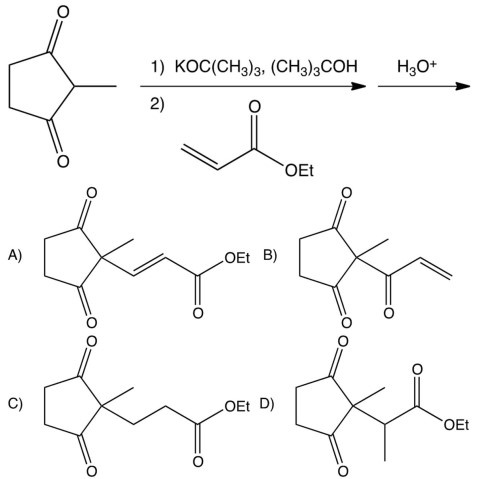
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
13
What would result from treating (-)-menthone with basic ?
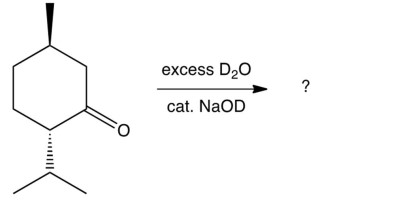
A)
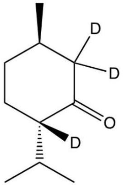
B)
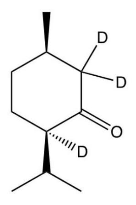
C)
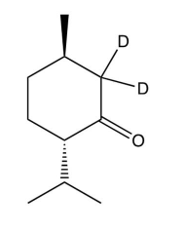
D) more than one of these is formed

A)

B)

C)

D) more than one of these is formed

Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck
14
The compound shown below undergoes a retro (reverse) aldol reaction under these conditions. What product(s) result(s)?
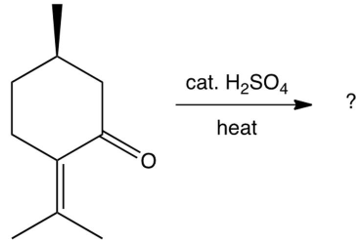
A)
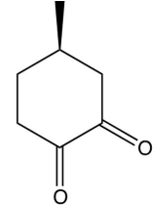
B)
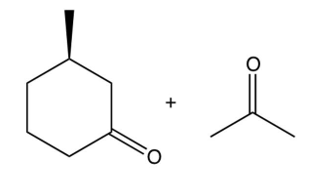
C)
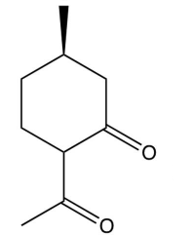
D)
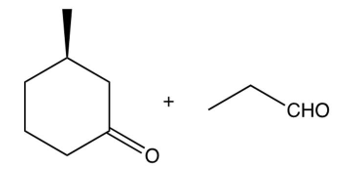

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 14 flashcards in this deck.
Unlock Deck
k this deck


