Deck 12: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 12: Chemical Kinetics
1
The following problem(s) pertain to The initial rate data given below for the reaction A2 2A. 
-Refer to Exhibit 18-1. What is the order of the reaction?
A) 0
B) 1
C) 2
D) Not enough data to answer the question
E) None of the above

-Refer to Exhibit 18-1. What is the order of the reaction?
A) 0
B) 1
C) 2
D) Not enough data to answer the question
E) None of the above
2
2
The initial rate data given below for the reaction A2 2A. 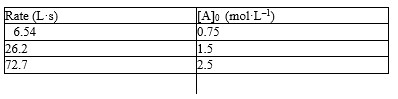
-Refer to Exhibit 18-1. What is the value of the rate constant?
A) .116
B) 11.6
C) 0.086
D) Not enough data to answer the question
E) None of the above

-Refer to Exhibit 18-1. What is the value of the rate constant?
A) .116
B) 11.6
C) 0.086
D) Not enough data to answer the question
E) None of the above
11.6
3
The initial rate data given below for the reaction A2 2A.
-Refer to Exhibit 18-1. What are the units for the rate constant?
A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) Not enough data to answer the question
F) None of the above
-Refer to Exhibit 18-1. What are the units for the rate constant?
A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) Not enough data to answer the question
F) None of the above
L·mol-1·s-1
4
The initial rate data given below for the reaction: 
- Refer to Exhibit 18-2. What is the order of the reaction with respect to AB, B, and C?
A) 1,1,1
B) 2,1,1
C) 1,2,1
D) 1,1,2
E) 1,3,1
F) Not enough data to answer the question

- Refer to Exhibit 18-2. What is the order of the reaction with respect to AB, B, and C?
A) 1,1,1
B) 2,1,1
C) 1,2,1
D) 1,1,2
E) 1,3,1
F) Not enough data to answer the question

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
The initial rate data given below for the reaction: 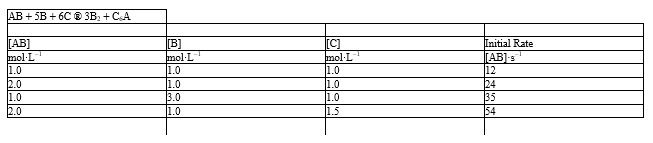
-Refer to Exhibit 18-2. What is the overall order of the reaction?
A) 1
B) 2
C) 3
D) 4
E) 5

-Refer to Exhibit 18-2. What is the overall order of the reaction?
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
The initial rate data given below for the reaction: 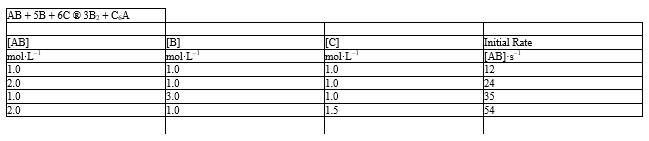
- Refer to Exhibit 18-2. What is the value of the rate constant?
A) 4
B) 3
C) 3.33
D) 18
E) None of the above

- Refer to Exhibit 18-2. What is the value of the rate constant?
A) 4
B) 3
C) 3.33
D) 18
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
The initial rate data given below for the reaction: 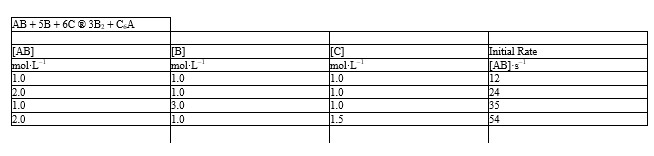
-Refer to Exhibit 18-2. What are the units of the rate constant?
A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) L3·mol-3·s-1
F) None of the above

-Refer to Exhibit 18-2. What are the units of the rate constant?
A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) L3·mol-3·s-1
F) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction of propene (C3CHCH2) with hydrochloric acid.
HCl + C3CHCH2
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_7ea7_87de_6b8f76fd28a4_TB10703_00.jpg) CH3CHClCH3
CH3CHClCH3
A proposed mechanism for this chemical reaction is
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_7ea8_87de_6319a3f2d31f_TB10703_00.jpg)
-Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?
A)![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_7ea9_87de_dfb5409d1dd4_TB10703_11.jpg)
B) k3[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above
HCl + C3CHCH2
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_7ea7_87de_6b8f76fd28a4_TB10703_00.jpg) CH3CHClCH3
CH3CHClCH3A proposed mechanism for this chemical reaction is
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_7ea8_87de_6319a3f2d31f_TB10703_00.jpg)
-Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?
A)
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_7ea9_87de_dfb5409d1dd4_TB10703_11.jpg)
B) k3[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
The reaction of propene (C3CHCH2) with hydrochloric acid.
HCl + C3CHCH2
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?</strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_a5ba_87de_bf878fc8f980_TB10703_11.jpg)
CH3CHClCH3
A proposed mechanism for this chemical reaction is
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?</strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_a5bb_87de_1bfb595603fe_TB10703_00.jpg)
-Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?
A)![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?</strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_a5bc_87de_712dffdfdfe6_TB10703_11.jpg)
B) k2[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above
HCl + C3CHCH2
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?</strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_a5ba_87de_bf878fc8f980_TB10703_11.jpg)
CH3CHClCH3
A proposed mechanism for this chemical reaction is
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?</strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_a5bb_87de_1bfb595603fe_TB10703_00.jpg)
-Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?
A)
![<strong>The reaction of propene (C<sub>3</sub>CHCH<sub>2</sub>) with hydrochloric acid. HCl + C<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub><sub> </sub> A proposed mechanism for this chemical reaction is -Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?</strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_a5bc_87de_712dffdfdfe6_TB10703_11.jpg)
B) k2[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction of propene (C3CHCH2) with hydrochloric acid.
HCl + C3CHCH2
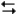
CH3CHClCH3
A proposed mechanism for this chemical reaction is
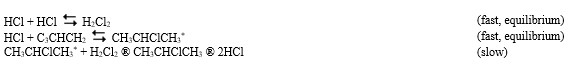
-Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?
A) 0
B) 1
C) 2
D) Experimental data required to answer this question
E) none of the above
HCl + C3CHCH2
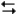
CH3CHClCH3
A proposed mechanism for this chemical reaction is
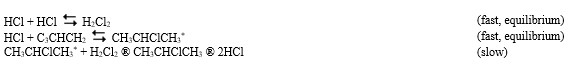
-Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?
A) 0
B) 1
C) 2
D) Experimental data required to answer this question
E) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction of propene (C3CHCH2) with hydrochloric acid.
HCl + C3CHCH2
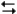
CH3CHClCH3
A proposed mechanism for this chemical reaction is

-Refer to Exhibit 18-3. Which of the species in the mechanism given above are reaction intermediates?
A) H2Cl2
B) HCl
C) CH3CHClCH3*
D) a and b
E) none of the above
HCl + C3CHCH2
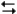
CH3CHClCH3
A proposed mechanism for this chemical reaction is

-Refer to Exhibit 18-3. Which of the species in the mechanism given above are reaction intermediates?
A) H2Cl2
B) HCl
C) CH3CHClCH3*
D) a and b
E) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
The following question(s) pertain to The scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.
![<strong>The following question(s) pertain to The scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) \rightarrow 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. - Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_ccd1_87de_e3e4f9fc7515_TB10703_00.jpg)
- Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?
A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.
![<strong>The following question(s) pertain to The scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) \rightarrow 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. - Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11eeda53_f152_ccd1_87de_e3e4f9fc7515_TB10703_00.jpg)
- Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?
A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck


