Deck 7: Thermodynamic Processes, Thermochemistry, Spontaneous Processes and Thermodynamic Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 7: Thermodynamic Processes, Thermochemistry, Spontaneous Processes and Thermodynamic Equilibrium
1
Calculate DS°rxn for the following
NH3(g) + HCl(g) NH4Cl(s)
A) 284.5 J·K-1
B) -284.5 J·K-1
C) -203.5 J·K-1
D) 89.1 J·K-1
NH3(g) + HCl(g) NH4Cl(s)
A) 284.5 J·K-1
B) -284.5 J·K-1
C) -203.5 J·K-1
D) 89.1 J·K-1
-284.5 J·K-1
2
Calculate DS°rxn for the following
3NO2(g) + H2O(l) 2HNO3(l) + NO(g)
A) 56.28 J·K-1
B) -56.28 J·K-1
C) 268.1 J·K-1
D) -268.1 J·K-1
3NO2(g) + H2O(l) 2HNO3(l) + NO(g)
A) 56.28 J·K-1
B) -56.28 J·K-1
C) 268.1 J·K-1
D) -268.1 J·K-1
-268.1 J·K-1
3
pertain to the reaction CCl4(l) CCl4(g).
-Refer to Exhibit 13-1. What is the value of DS°vap?
A) 93.3 J·K-1
B) -93.3 J·K-1
C) 32.5 J·K-1
D) -32.5 J·K-1
E) None of the above
-Refer to Exhibit 13-1. What is the value of DS°vap?
A) 93.3 J·K-1
B) -93.3 J·K-1
C) 32.5 J·K-1
D) -32.5 J·K-1
E) None of the above
93.3 J·K-1
4
Allotropic forms of an element that do not represent an element in its standard state will have
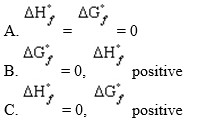
D) S°=0
E) None of the above
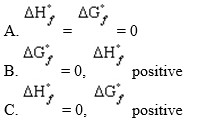
D) S°=0
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
A solid of mass m initially at T0 is heated to T1 at constant pressure where it proceeds to melt. Once all of the solid is in the liquid phase it is further heated to T2. If the solid and liquid have specific heat capacities of cpsol and cpsol respectively, which equation below best represents the change in entropy for this system?
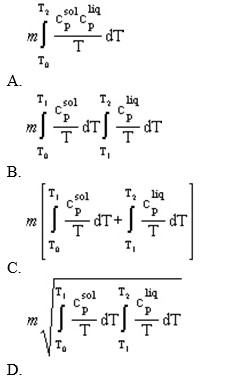
E) B and D

E) B and D

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
The constant that connects the entropy of a system to the number of microstates is
A) R
B)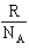
C) kB
D)

E) B and C
A) R
B)
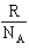
C) kB
D)

E) B and C

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
For a reversible isothermal process involving ideal gases
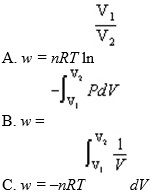
D) b and c
E) all of the above
F) none of the above
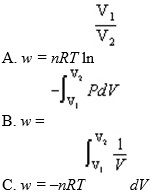
D) b and c
E) all of the above
F) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
Copper has a specific heat capacity of 0.0920 cal·K-1·g-1 at 298 K. What is the molar heat capacity of copper in J·K-1·mol-1?
A) 22.6 J·K-1·mol-1
B) 24.5 J·K-1·mol-1
C) 1.40 J·K-1·mol-1
D) 3.46´10-4 J·K-1·mol-1
E) None of the above
A) 22.6 J·K-1·mol-1
B) 24.5 J·K-1·mol-1
C) 1.40 J·K-1·mol-1
D) 3.46´10-4 J·K-1·mol-1
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
A 125.0 g piece of iron is heated to 400°C and placed in 300.0 g of water at 25°C. If the specific heat capacities of iron and water are 0.449 J·K-1·g-1 and 4.184 J·K-1·g-1 respectively, what will be the final temperature of the water?
A) 32°C
B) 41°C
C) 101°C
D) 383°C
E) None of the above
A) 32°C
B) 41°C
C) 101°C
D) 383°C
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
The decomposition of NO and NO2 to the elements proceeds via the following two reactions.  Calculate the enthalpy change for the reaction
Calculate the enthalpy change for the reaction
2NO(g) + O2(g) 2NO2(g)
A) 244 kJ
B) -244 kJ
C) -114 kJ
D) -293 kJ
E) none of the above
 Calculate the enthalpy change for the reaction
Calculate the enthalpy change for the reaction2NO(g) + O2(g) 2NO2(g)
A) 244 kJ
B) -244 kJ
C) -114 kJ
D) -293 kJ
E) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
Sublimation is the process of changing phases directly from a solid to a gas. Calculate DH°sub for elemental iodine.
A) 62.44 kJ·mol-1
B) -62.44 kJ·mol-1
C) 39.98 kJ·mol-1
D) 19.36 kJ·mol-1
A) 62.44 kJ·mol-1
B) -62.44 kJ·mol-1
C) 39.98 kJ·mol-1
D) 19.36 kJ·mol-1

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
The standard state of carbon as a free element is graphite. C60 is an allotropic form of carbon belonging to a class of structures known as fullerenes. 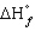 for C60 should be
for C60 should be
A) zero
B) positive
C) negative
D) equal to
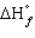 for the other allotropic forms of carbon
for the other allotropic forms of carbon
E) A and D
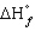 for C60 should be
for C60 should beA) zero
B) positive
C) negative
D) equal to
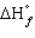 for the other allotropic forms of carbon
for the other allotropic forms of carbonE) A and D

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck


