Deck 6: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 6: Solutions
1
96.5 grams of an unknown substance were diluted with water in a 1.000 L volumetric flask. It was determined that the concentration of the substance was 1.22 M. What is the molar mass of the unknown substance?
A) 117 g·mol-1
B) 79.1 g·mol-1
C) 1.26´10-2 g·mol-1
D) 65.4 g·mol-1
A) 117 g·mol-1
B) 79.1 g·mol-1
C) 1.26´10-2 g·mol-1
D) 65.4 g·mol-1
79.1 g·mol-1
2
Equal volumes of ethanol (C2H5OH) and water are mixed. If the density of water is 1.00 g·cm-3 and that of ethanol is 0.789, what is the mole fraction of ethanol in the mixture?
A) 0.764
B) 0.309
C) 0.324
D) 0.236
A) 0.764
B) 0.309
C) 0.324
D) 0.236
0.236
3
120.0 grams of calcium nitrate are weighed out and placed into a 1.5 L volumetric flask and the flask filled to the mark. What is the concentration of the nitrate ions in solution?
A) 0.9751
B) 0.4875
C) 1.026
D) 2.051
A) 0.9751
B) 0.4875
C) 1.026
D) 2.051
0.9751
4
How many ml of 0.1506 M NaOH are required to reach the endpoint in a titration with 30.00 ml of 0.2215 M HCl?
A) 20.40
B) 30.15
C) 31.18
D) 44.12
A) 20.40
B) 30.15
C) 31.18
D) 44.12

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
Which, if any, of the following would you expect to readily dissolve in water?
A)
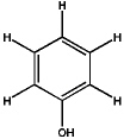
B)
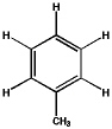
C)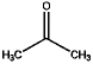
D) a and c
E) none of the above
A)

B)

C)

D) a and c
E) none of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
What is the expected boiling point of a solution made by combining 400.0 g of water with 100.0 g of Na2CO3?
A) 103.6°C
B) 113.5°C
C) 101.2°C
D) 104.8°C
A) 103.6°C
B) 113.5°C
C) 101.2°C
D) 104.8°C

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
The following question(s) pertain to the dissolution of 200.0 g of KMnO4 into 600.0 g. of water.
-Refer to Exhibit 11-2. What is the molality of the KMnO4 in the 800.0 gram solution?
A) 1.255 m
B) 2.109 m
C) 1.054 m
D) 2.223 m
-Refer to Exhibit 11-2. What is the molality of the KMnO4 in the 800.0 gram solution?
A) 1.255 m
B) 2.109 m
C) 1.054 m
D) 2.223 m

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
The following question(s) pertain to the dissolution of 200.0 g of KMnO4 into 600.0 g. of water.
-Refer to Exhibit 11-2. What is the mole fraction of MnO4- ions in the solution?
A) 0.0353
B) 0.0365
C) 0.0433
D) 0.0333
-Refer to Exhibit 11-2. What is the mole fraction of MnO4- ions in the solution?
A) 0.0353
B) 0.0365
C) 0.0433
D) 0.0333

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
pertain to the dissolution of 200.0 g of KMnO4 into 600.0 g. of water.
- Refer to Exhibit 11-2. If 200.0 ml of a solution that was 0.20 M in KMnO4 were added to the 800.0 g solution, would you expect the boiling point of that solution to go up?
A) Yes
B) No
C) More data is needed to answer the question
- Refer to Exhibit 11-2. If 200.0 ml of a solution that was 0.20 M in KMnO4 were added to the 800.0 g solution, would you expect the boiling point of that solution to go up?
A) Yes
B) No
C) More data is needed to answer the question

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
To prepare 0.250 L of a 0.115M NaBr solution, a technician must mix
A) 0.115 moles of NaBr with 0.250 L of H2O.
B) 0.115 moles of NaBr with 0.250 kg of H2O.
C) 0.115 moles of NaBr with enough H2O to produce 0.250 kg of solution.
D) 0.115 moles of NaBr with enough H2O to produce 0.250 L of solution.
E) none of these
A) 0.115 moles of NaBr with 0.250 L of H2O.
B) 0.115 moles of NaBr with 0.250 kg of H2O.
C) 0.115 moles of NaBr with enough H2O to produce 0.250 kg of solution.
D) 0.115 moles of NaBr with enough H2O to produce 0.250 L of solution.
E) none of these

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
320 mL of 1.00 M HCl(aq) is combined with 1.18 L of 0.125 M HCl(aq) to give a total volume of 1.50 L. The molarity of the HCl in the final solution is
A) 0.562 M
B) 0.468 M
C) 0.312 M
D) 0.175 M
E) none of these
A) 0.562 M
B) 0.468 M
C) 0.312 M
D) 0.175 M
E) none of these

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
Acetone (mw = 58.08, P 25°C =232 mmHg) and butanone (mw = 72.11, P 25 °C =100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
A) 5.313 kg
B) 290 g
C) 188 g
D) More information needed
E) None of the above
A) 5.313 kg
B) 290 g
C) 188 g
D) More information needed
E) None of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck


