Deck 1: The Atom in Modern Chemistry
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/7
Play
Full screen (f)
Deck 1: The Atom in Modern Chemistry
1
Two elements X and Y combine to form two compounds with the following compositions 
If compound 1 has the formula XY3 then a possible formula for compound 2 is
A) XY2
B) X2Y5
C) XY4
D) X2Yl9
E) XY5

If compound 1 has the formula XY3 then a possible formula for compound 2 is
A) XY2
B) X2Y5
C) XY4
D) X2Yl9
E) XY5
XY5
2
Gibbsite is an aluminum ore that is composed of 34.59% Al, 3.88% H, and 61.53% O. How much aluminum can be refined from 248.0 g of Gibbsite?
A) 7.09 g
B) 22.81 g
C) 45.59 g
D) 85.78 g
E) 152.6 g
A) 7.09 g
B) 22.81 g
C) 45.59 g
D) 85.78 g
E) 152.6 g
85.78 g
3
The element zinc has two naturally occurring isotopes. The natural abundances and isotopic masses are 
The atomic mass of naturally occurring zinc is
A) 112.990
B) 113.582
C) 113.904
D) 114.582
E) 114.818

The atomic mass of naturally occurring zinc is
A) 112.990
B) 113.582
C) 113.904
D) 114.582
E) 114.818
114.818
4
The element antinomy has two naturally occurring isotopes. The natural abundances and isotopic masses are 
The atomic mass of naturally occurring antinomy is
A) 121.760
B) 121.904
C) 122.048
D) 122.904
E) 124.048

The atomic mass of naturally occurring antinomy is
A) 121.760
B) 121.904
C) 122.048
D) 122.904
E) 124.048

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
5
If the density of the nucleus of gold is approximately 1´1015 g cm-3 and solid gold has a density of 19.3 g cm-3 estimate the percentage of the volume of solid gold that is "occupied" by the nucleus?
A) 2´10-12 %
B) 2´10-8 %
C) 2´10-4 %
D) 2´10-2 %
E) 2%
A) 2´10-12 %
B) 2´10-8 %
C) 2´10-4 %
D) 2´10-2 %
E) 2%

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
6
In a strange and alternative universe you repeat Millikan's oil drop experiment and measure the following charges on different oil drops 
Using this give your best estimate for the unit of fundamental charge in this alternative universe
A) 1.2´10-17 C
B) 1.8´10-17 C
C) 3.6´10-17 C
D) 9.0´10-17 C
E) 1.2´10-16 C

Using this give your best estimate for the unit of fundamental charge in this alternative universe
A) 1.2´10-17 C
B) 1.8´10-17 C
C) 3.6´10-17 C
D) 9.0´10-17 C
E) 1.2´10-16 C

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
7
Which nuclide has more protons than neutrons?
A)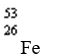
B)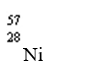
C)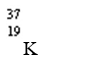
D)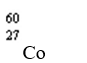
A)
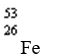
B)
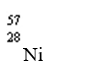
C)
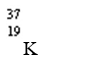
D)
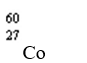

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck


