Deck 15: Distillation Systems
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 15: Distillation Systems
1
Two basic tower designs include:
A) packed and tray
B) plate and packed
C) bubblecap and sieve
D) valve and packed
E) a & b
A) packed and tray
B) plate and packed
C) bubblecap and sieve
D) valve and packed
E) a & b
B
2
Overlap can be minimized by:
A) refluxing
B) increasing pressure
C) reboiling
D) a & c
A) refluxing
B) increasing pressure
C) reboiling
D) a & c
D
3
High bottoms temperature in a distillation tower are controlled by :
A) increase over head flow and decrease bottoms flow
B) decrease overhead flow and increase bottoms flow
C) stop feed flow and cut bottom temperature
D) open side-streams
A) increase over head flow and decrease bottoms flow
B) decrease overhead flow and increase bottoms flow
C) stop feed flow and cut bottom temperature
D) open side-streams
A
4
List and describe the three technology periods of distillation.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
Feed composition changes have very little impact on the energy balance but significant impact on the material balance.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the total vapor pressure exerted by the mixture: 25% hexane 20.6 psia @ 175F, 25% heptane 8.8 psia @ 175F, and 50% benzene 14.7 psia @ 176 F. Give answer in psia.
A) 14.7 psia
B) 44.1 psia
C) 22.05 psia
D) 34.7 psig
A) 14.7 psia
B) 44.1 psia
C) 22.05 psia
D) 34.7 psig

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Puking is most closely related to:
A) reduced partial pressure
B) excessive vapor pressure
C) low temperature
D) high temperature
A) reduced partial pressure
B) excessive vapor pressure
C) low temperature
D) high temperature

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
The process of vaporizing liquids at lower temperatures by reducing pressure:
A) azeotropic distillation
B) fractional distillation
C) vacuum distillation
D) adsorption distillation
A) azeotropic distillation
B) fractional distillation
C) vacuum distillation
D) adsorption distillation

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Tube shaped funnels that allow fluid transfer between plates in a tower:
A) riser
B) bubble-cap
C) downcomer
D) sieve
A) riser
B) bubble-cap
C) downcomer
D) sieve

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
The temperature at which the heaviest component begins to boil:
A) IBP- initial boiling point
B) FBP- final boiling point
C) ABP- average boiling point
D) Dew point
A) IBP- initial boiling point
B) FBP- final boiling point
C) ABP- average boiling point
D) Dew point

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
Excessive liquid flowing down a tower that blocks vapor flow up the tower:
A) puking
B) flooding
C) surging
D) reflux
A) puking
B) flooding
C) surging
D) reflux

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
A binary mixture contains:
A) lighter hydrocarbons
B) is heavier than a ternary mixture
C) two or more components
D) three compounds in a mixture
A) lighter hydrocarbons
B) is heavier than a ternary mixture
C) two or more components
D) three compounds in a mixture

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
Boil-up rate is defined as:
A) the temperature at which a liquid turns to vapor
B) the vapor-liquid balance returned to tower from the reboiler
C) the vapor-liquid reflux balance returned to tower from overhead accumulator.
D) the total amount of vapor moving up the column.
A) the temperature at which a liquid turns to vapor
B) the vapor-liquid balance returned to tower from the reboiler
C) the vapor-liquid reflux balance returned to tower from overhead accumulator.
D) the total amount of vapor moving up the column.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
Draw a distillation tower system. Include (3) three control loops, preheater, condenser, kettle reboiler, distillation column.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
What is the composition of side stream #2z?
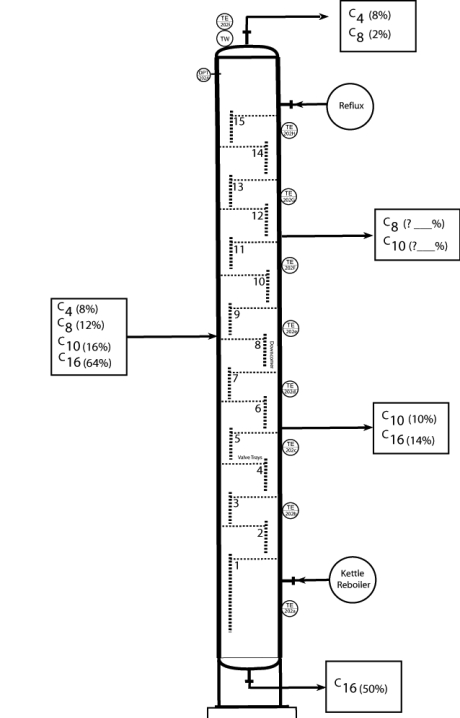


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
The sum of the products entering the column equals the sum leaving:
A) energy balance
B) fractional balance
C) material balance
D) total balance
A) energy balance
B) fractional balance
C) material balance
D) total balance

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
Packed towers use specially designed packing materials to reduce surface area during the distillation process:

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
Distillation columns have two major types of separation grids: plate and packing. Plate towers have the following tray types:

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
Packed towers have the following types of packing:

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
Liquid distillate returned to the tower is called:
A) boil-up rate
B) reflux rate
C) total reflux
D) incomplete separation
A) boil-up rate
B) reflux rate
C) total reflux
D) incomplete separation

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
The temperature at which a liquid mixture turns to vapor:
A) boil-up rate
B) IBP
C) FBP
D) boiling point
E) dew point
A) boil-up rate
B) IBP
C) FBP
D) boiling point
E) dew point

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
What is the overhead composition?
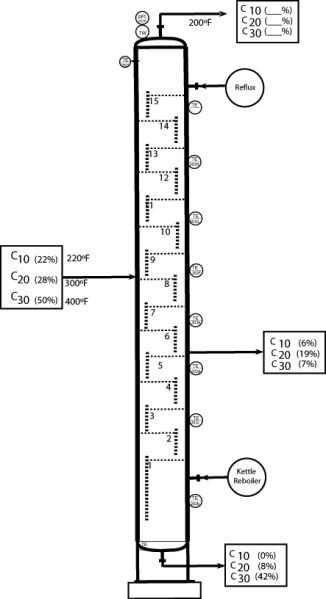


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
The upper section of the tower is referred to as:
A) feed section
B) rectifying section
C) enriching section
D) stripping section
E) b & c
A) feed section
B) rectifying section
C) enriching section
D) stripping section
E) b & c

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
Condensed tower product that is pumped back into a tower to increase product purity and control temperature:
A) overlap
B) reflux
C) side-stream
D) accumulation
A) overlap
B) reflux
C) side-stream
D) accumulation

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
The condensed gas taken from a distillation tower is called:
A) residuum
B) distillate
C) condensate
D) distillation
A) residuum
B) distillate
C) condensate
D) distillation

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
Distillation is best defined by the following statement:
A) the separation of components in a mixture by tower segregation
B) the fractional separation of a binary mixture by tray filtration
C) the azeotropic process designed to separate hydrocarbons by fractional boiling points.
D) the separation of components in a mixture by boiling point
A) the separation of components in a mixture by tower segregation
B) the fractional separation of a binary mixture by tray filtration
C) the azeotropic process designed to separate hydrocarbons by fractional boiling points.
D) the separation of components in a mixture by boiling point

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
The lower section of the tower is referred to as:
A) feed section
B) rectifying section
C) enriching section
D) stripping section
E) b & c
A) feed section
B) rectifying section
C) enriching section
D) stripping section
E) b & c

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
The tray located below the inlet feed line is called the:
A) mid section tray
B) pumping section tray
C) feed tray
D) valve tray
A) mid section tray
B) pumping section tray
C) feed tray
D) valve tray

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
Each tray in a distillation column has a different molecular structure.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
The varying temperatures across the tower:
A) temperature gradient
B) temperature profile
C) temperature overlap
D) temperature delta
A) temperature gradient
B) temperature profile
C) temperature overlap
D) temperature delta

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
List three process variables that can be used to break a flood:

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
List the critical steps used to start-up a distillation system. (DEXTER?)

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
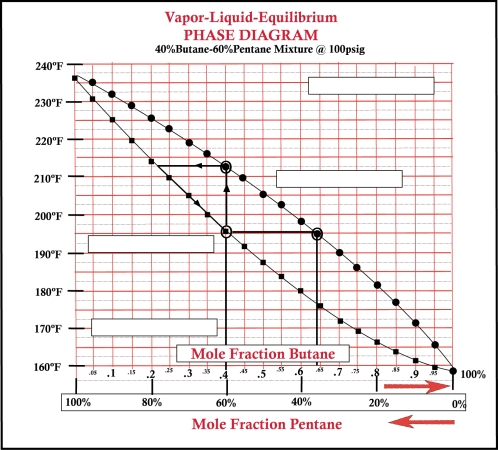
-Refer to Bottom VLE graphic above. On a VLE chart the bottom curve is referred to as:_________________ .

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
A ternary mixture:
A) lighter hydrocarbons
B) is heavier than a ternary mixture
C) two or more components
D) three compounds in a mixture
A) lighter hydrocarbons
B) is heavier than a ternary mixture
C) two or more components
D) three compounds in a mixture

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
When a heat exchanger is used to convert all of the overhead vapors to liquid, it is called:
A) normal overhead condenser
B) total reflux
C) total condenser
D) partial condenser
A) normal overhead condenser
B) total reflux
C) total condenser
D) partial condenser

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
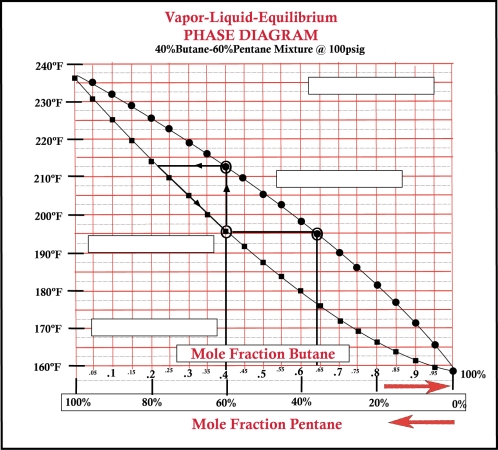
-Refer to Bottom VLE graphic above. On a VLE chart the top curve is referred to as:_________________ .

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
All of the following are principles of distillation except:
A) heat increases vapor pressure
B) pressure increases boiling point
C) vacuum decreases boiling point
D) forms and breaks molecular bonds
A) heat increases vapor pressure
B) pressure increases boiling point
C) vacuum decreases boiling point
D) forms and breaks molecular bonds

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
The larger the difference between partial pressures the harder it is to separate the fractions by boiling point.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
Using information from question 6, calculate the % component in vapor of hexane:
A) 20%
B) 25%
C) 30%
D) 35%
A) 20%
B) 25%
C) 30%
D) 35%

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
Vapor distillate returned to the tower is called:
A) boil-up rate
B) reflux rate
C) total reflux
D) incomplete separation
A) boil-up rate
B) reflux rate
C) total reflux
D) incomplete separation

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
The vapor medium suspended above a boiling mixture is enriched with the components that have the lowest boiling points.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Heat that cannot be sensed or measured:
A) latent
B) sensible
C) kinetic
D) potential energy
A) latent
B) sensible
C) kinetic
D) potential energy

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Incomplete separation of components in a mixture:
A) overlap
B) puking
C) temperature gradient
D) overloading
A) overlap
B) puking
C) temperature gradient
D) overloading

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
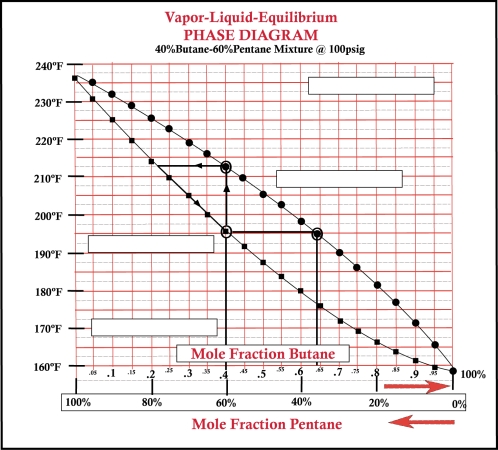
-Refer to Bottom VLE graphic above. The initial bubble point temperature for a butane mole fraction of 40% is:_________________ .

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
The temperature at which a liquid boils and forms vapors is referred to as the:
A) boiling point
B) specific temperature
C) bubble point
D) dew point
A) boiling point
B) specific temperature
C) bubble point
D) dew point

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
The Vapor Liquid Equilibrium diagram is primarily used for :
A) a condition or state where the rate of evaporation (liquid changing to vapor) equals the rate of condensation (vapor changing to a liquid).
B) A & C
C) visualizing a condition where a liquid and it's vapor (gas phase) are in equilibrium with each other.
D) components in a binary mixture exert partial pressures that are equal.
A) a condition or state where the rate of evaporation (liquid changing to vapor) equals the rate of condensation (vapor changing to a liquid).
B) A & C
C) visualizing a condition where a liquid and it's vapor (gas phase) are in equilibrium with each other.
D) components in a binary mixture exert partial pressures that are equal.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
The Reflux Ratio is best defined as :
A) the amount of overhead product returned to the column as reflux in relation to the amount of overhead taken off to storage.
B) careful control of column top temperature and product purity.
C) reflux rate divided by product rate
D) A & C
A) the amount of overhead product returned to the column as reflux in relation to the amount of overhead taken off to storage.
B) careful control of column top temperature and product purity.
C) reflux rate divided by product rate
D) A & C

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck


