Deck 7: The Reactions of Alkynes an Introduction Tomultistep Synthesis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 7: The Reactions of Alkynes an Introduction Tomultistep Synthesis
1
What is the systematic name for the following compound? ? 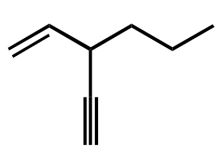
A) 3-ethynyl-1-hexene
B) 3-ethenyl-1-hexyne
C) 3-propyl-4-penten-1-yne
D) 3-propyl-1-penten-4-yne
E) 3-propyl-1,4-pentenyne

A) 3-ethynyl-1-hexene
B) 3-ethenyl-1-hexyne
C) 3-propyl-4-penten-1-yne
D) 3-propyl-1-penten-4-yne
E) 3-propyl-1,4-pentenyne
3-propyl-1-penten-4-yne
2
What is the systematic name for the following compound? 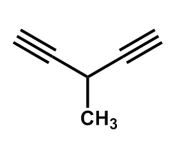
A) 1,1-diethynylethane
B) 3-ethynylbutyne
C) 3-butynylethyne
D) 3-ethynyl-1-butyne
E) 3-methyl-1,4-pentadiyne

A) 1,1-diethynylethane
B) 3-ethynylbutyne
C) 3-butynylethyne
D) 3-ethynyl-1-butyne
E) 3-methyl-1,4-pentadiyne
3-methyl-1,4-pentadiyne
3
Why are alkynes less reactive toward electrophilic addition?
A) Triple bonds are stronger than double bonds.
B) Only one ? bond must be broken in the addition of an electrophile to an alkene.
C) The ?-complex that is formed from an alkyne is less stable than the alkyl cation intermediate that is formed from an alkene.
D) Alkynes are less stable than alkenes.
E) Hyperconjugation stabilizes the intermediate from an alkyne more than the intermediate from an alkene.
A) Triple bonds are stronger than double bonds.
B) Only one ? bond must be broken in the addition of an electrophile to an alkene.
C) The ?-complex that is formed from an alkyne is less stable than the alkyl cation intermediate that is formed from an alkene.
D) Alkynes are less stable than alkenes.
E) Hyperconjugation stabilizes the intermediate from an alkyne more than the intermediate from an alkene.
The ?-complex that is formed from an alkyne is less stable than the alkyl cation intermediate that is formed from an alkene.
4
Which product is expected from the reaction shown here?
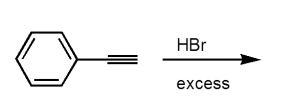
A)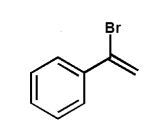
B)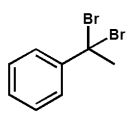
C)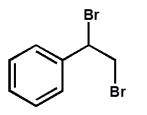
D)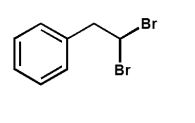
E)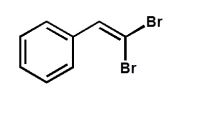
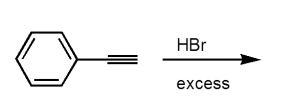
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
Which product would be isolated from the reaction shown?
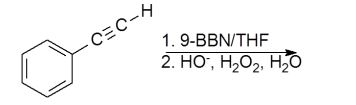
A)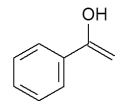
B)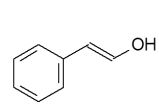
C)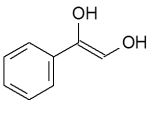
D)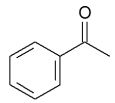
E)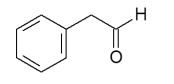

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
Which reagent is the best choice to complete the following transformation?
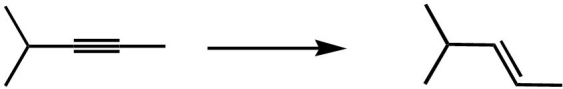
A) Na, NH3(liq)
B) NaNH2
C) 1. NaBH4, 2. H3O+
D) 1 mole H2/Pd/C
E) H2, Lindlar catalyst

A) Na, NH3(liq)
B) NaNH2
C) 1. NaBH4, 2. H3O+
D) 1 mole H2/Pd/C
E) H2, Lindlar catalyst

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements is not true?
A) Alkynes undergo electrophilic addition reactions.
B) Alkynes are less reactive than alkenes in electrophilic addition reactions.
C) Sodium in liquid ammonia is used to form cis alkenes.
D) Catalytic hydrogenation of an alkyne in the presence of Pd/C gives complete reduction.
E) Reaction of an alkyne with water under acidic conditions gives an enol.
A) Alkynes undergo electrophilic addition reactions.
B) Alkynes are less reactive than alkenes in electrophilic addition reactions.
C) Sodium in liquid ammonia is used to form cis alkenes.
D) Catalytic hydrogenation of an alkyne in the presence of Pd/C gives complete reduction.
E) Reaction of an alkyne with water under acidic conditions gives an enol.

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
Which base is strong enough to deprotonate 1-octyne completely?
A) NaF
B) NaOH
C) NH3
D) NaOCH3
E) NaNH2
A) NaF
B) NaOH
C) NH3
D) NaOCH3
E) NaNH2

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following methods would give the product in reasonable yield? 
A) 1. NaNH2 2. CH2=CHCH2Br 3. H2O, H2SO4
B) 1. NaNH2 2. CH2=CHCH2Br 3. HCl
C) 1. NaNH2 2. CH2=CHCH2Br 3. R2BH/THF 4. HO?,H2O2, H2O
D) 1. NaNH2 2. HOCH2CHCH2Br 3. R2BH/THF 4. HO?, H2O2, H2O
E) all of the above

A) 1. NaNH2 2. CH2=CHCH2Br 3. H2O, H2SO4
B) 1. NaNH2 2. CH2=CHCH2Br 3. HCl
C) 1. NaNH2 2. CH2=CHCH2Br 3. R2BH/THF 4. HO?,H2O2, H2O
D) 1. NaNH2 2. HOCH2CHCH2Br 3. R2BH/THF 4. HO?, H2O2, H2O
E) all of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following sets of reactions accomplish the synthesis shown below? 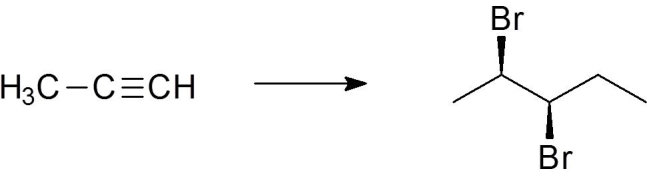
A) 1. NaNH2 2. CH3CH2CH2Br 3. Na/NH3(liq) 4. HBr
B) 1. NaOH 2. CH3CH2Br 3. H2, Lindlar cat 4. Br2/H2O
C) 1. NaNH2 2. CH3CH2I 3. H2, Lindlar cat 4. Br2
D) 1. H2, Lindlar cat 2. NaNH2 3. CH3CH2Br 4. Br2
E) 1. Na/NH3(liq) 2. NaNH2 3. CH3CH2I 4. HBr

A) 1. NaNH2 2. CH3CH2CH2Br 3. Na/NH3(liq) 4. HBr
B) 1. NaOH 2. CH3CH2Br 3. H2, Lindlar cat 4. Br2/H2O
C) 1. NaNH2 2. CH3CH2I 3. H2, Lindlar cat 4. Br2
D) 1. H2, Lindlar cat 2. NaNH2 3. CH3CH2Br 4. Br2
E) 1. Na/NH3(liq) 2. NaNH2 3. CH3CH2I 4. HBr

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following sets of reactions accomplishes the synthesis shown below? 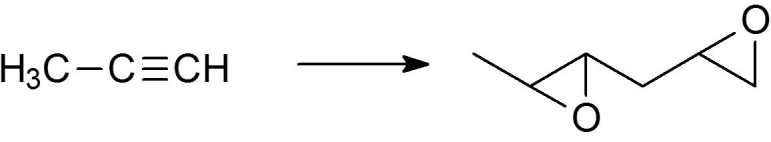
A) 1. NaNH2 2. CH2=CHCH2Br 3. H2, Lindlar 4. BH3/THF 5. HO?,H2O2, H2O
B) 1. NaNH2 2. CH2=CHCH2Br 3. MCPBA
C) 1. NaNH2 2. CH3CH2CH2Br 3. BH3/THF 4. HO?,H2O2, H2O
D) 1. NaNH2 2. CH2=CHCH2Br 3. Na/NH3(liq) 4. MCPBA
E) 1. NaNH2 2. CH3CH2CH2Br 4. 9-BBN-THF 5. HO-,H2O2, H2O

A) 1. NaNH2 2. CH2=CHCH2Br 3. H2, Lindlar 4. BH3/THF 5. HO?,H2O2, H2O
B) 1. NaNH2 2. CH2=CHCH2Br 3. MCPBA
C) 1. NaNH2 2. CH3CH2CH2Br 3. BH3/THF 4. HO?,H2O2, H2O
D) 1. NaNH2 2. CH2=CHCH2Br 3. Na/NH3(liq) 4. MCPBA
E) 1. NaNH2 2. CH3CH2CH2Br 4. 9-BBN-THF 5. HO-,H2O2, H2O

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
Rank the following carbocations in order of decreasing stability.
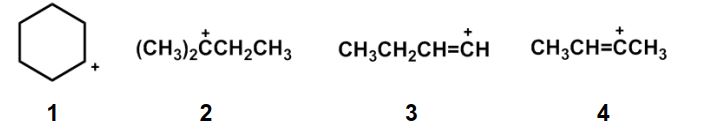
A) 2 > 1 > 4 > 3
B) 2>1>3>4
C) 4>3>2>1
D) 3>4>2>1
E) 2>4>3>1
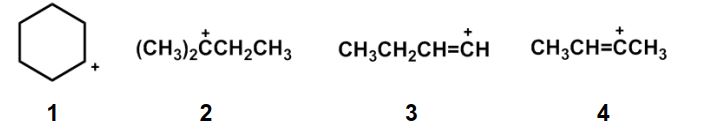
A) 2 > 1 > 4 > 3
B) 2>1>3>4
C) 4>3>2>1
D) 3>4>2>1
E) 2>4>3>1

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck


