Deck 5: Alkenes Structure, Nomenclature, and an Introduction to Reactivity Thermodynamics and Kinetics
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/8
Play
Full screen (f)
Deck 5: Alkenes Structure, Nomenclature, and an Introduction to Reactivity Thermodynamics and Kinetics
1
What is the degree of unsaturation for a compound with molecular formula
C23H38?
A) 4
B) 5
C) 7.5
D) 8
E) 15
C23H38?
A) 4
B) 5
C) 7.5
D) 8
E) 15
5
2
What is the correct name for the following compound?
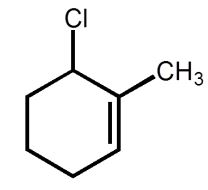
A) 1-methyl-2-chlorocylohex-2-ene
B) 1-chloro-2-methylcylohex-2-ene
C) 2-methyl-3-chlorocyclohexene
D) 6-chloro-1-methylcylohexene
E) 3-chloro-2-methylcyclohexene

A) 1-methyl-2-chlorocylohex-2-ene
B) 1-chloro-2-methylcylohex-2-ene
C) 2-methyl-3-chlorocyclohexene
D) 6-chloro-1-methylcylohexene
E) 3-chloro-2-methylcyclohexene
6-chloro-1-methylcylohexene
3
Which of the following alkenes has the greatest heat of hydrogenation? 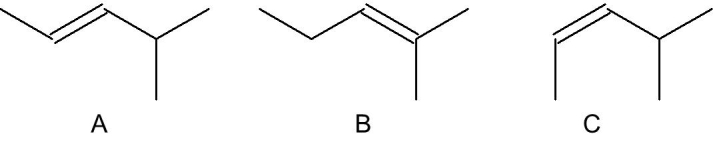
A) A
B) B
C) C
D) all are about the same
E) A and B have the greatest

A) A
B) B
C) C
D) all are about the same
E) A and B have the greatest
C
4
Which of the following are nucleophiles? 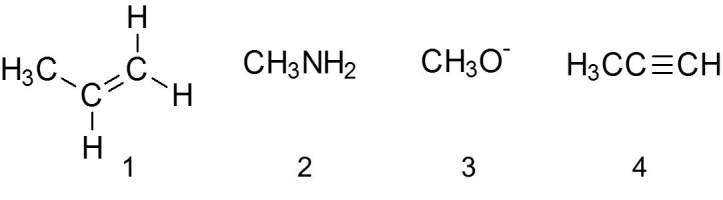
A) 1
B) 2
C) 3
D) 4
E) all of the above

A) 1
B) 2
C) 3
D) 4
E) all of the above

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
5
The rate of a chemical reaction depends on which of the following?
A) the concentration of reactants
B) the temperature at which the reaction is carried out
C) the fraction of collisions that occur with the proper orientation
D) the presence of a catalyst
E) all of the above
A) the concentration of reactants
B) the temperature at which the reaction is carried out
C) the fraction of collisions that occur with the proper orientation
D) the presence of a catalyst
E) all of the above

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is correct?
A) The ?G° of a reaction depends on the mechanism (i.e., the path).
B) The ?G‡ of a reaction depends on the mechanism.
C) The ?G° of reactions may be changed by adding a catalyst.
D) The bigger the ?G°, the faster the reaction.
E) The bigger the ?G°, the slower the reaction.
A) The ?G° of a reaction depends on the mechanism (i.e., the path).
B) The ?G‡ of a reaction depends on the mechanism.
C) The ?G° of reactions may be changed by adding a catalyst.
D) The bigger the ?G°, the faster the reaction.
E) The bigger the ?G°, the slower the reaction.

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about catalysts is false?
A) A catalyst decreases the activation energy of a reaction.
B) A catalyst does not change the energy of the reactants or products.
C) A catalyst increases the amount of product formed.
D) A catalyst increases the rate of a reaction.
E) A catalyst is not consumed in a reaction.
A catalyst changes only the rate at which the product is formed, not the amount of product formed.
A) A catalyst decreases the activation energy of a reaction.
B) A catalyst does not change the energy of the reactants or products.
C) A catalyst increases the amount of product formed.
D) A catalyst increases the rate of a reaction.
E) A catalyst is not consumed in a reaction.
A catalyst changes only the rate at which the product is formed, not the amount of product formed.

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
8
Rank the following compounds in order from most stable to least stable. 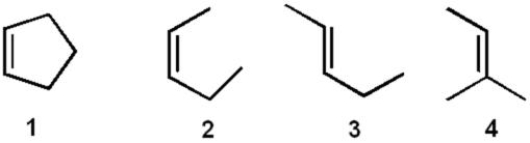
A) 1>2>3>4
B) 1>2>4>3
C) 4>1>2>3
D)4>3>2>1
E) 2>4>1>3

A) 1>2>3>4
B) 1>2>4>3
C) 4>1>2>3
D)4>3>2>1
E) 2>4>1>3

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck


