Deck 4: Isomers the Arrangement of Atoms in Space
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 4: Isomers the Arrangement of Atoms in Space
1
How many asymmetric carbon centers are found in cabergoline, a drug used to treat Parkinson's disease?
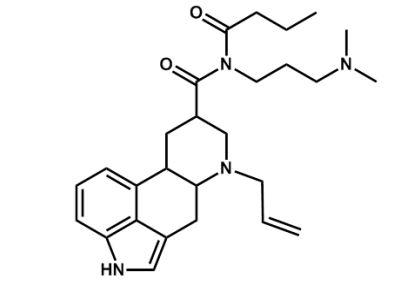
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5
3
2
Which of the following structures is an E isomer?
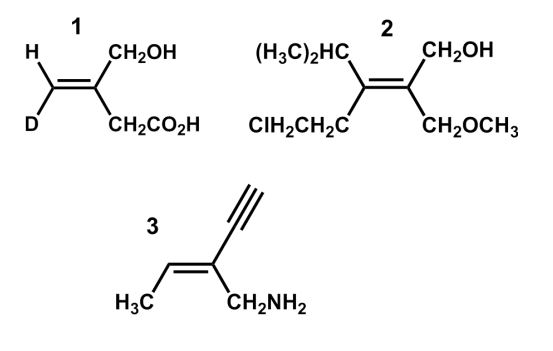
A) 1
B) 2
C) 3
D) 1 and 2
E) 1 and 3

A) 1
B) 2
C) 3
D) 1 and 2
E) 1 and 3
1 and 2
3
A sample of the disulfide L-cystine has an observed specific rotation of -14. Pure L-cystine has a specific rotation of -70. What percent of each enantiomer is present in the sample?
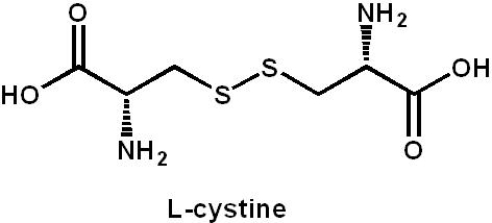
A) 14 (?) and 56 (+)
B) 20 (?) and 80 (+)
C) 60 (?) and 40 (+)
D) 80 (?) and 20 (+)
E) 84 (?) and 16 (+)

A) 14 (?) and 56 (+)
B) 20 (?) and 80 (+)
C) 60 (?) and 40 (+)
D) 80 (?) and 20 (+)
E) 84 (?) and 16 (+)
60 (?) and 40 (+)
4
Which structure(s) is(are) an enantiomer of the molecule at the top left?
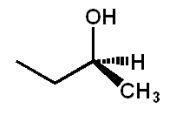
A)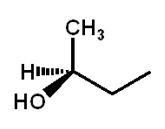
B)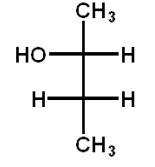
C)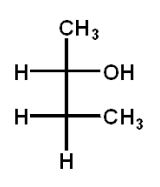
D)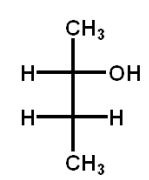

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following structures has the S configuration?
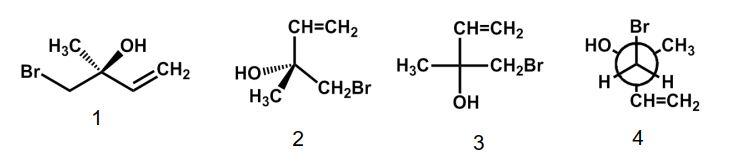
A) 1
B) 2
C) 3
D) 4
E) 1 and 3

A) 1
B) 2
C) 3
D) 4
E) 1 and 3

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
What is the relationship between the two compounds shown below? 
A) enantiomers
B) diastereomers
C) constitutional isomers
D) geometric isomers
E) identical compounds

A) enantiomers
B) diastereomers
C) constitutional isomers
D) geometric isomers
E) identical compounds

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following structures has the S,S configuration?
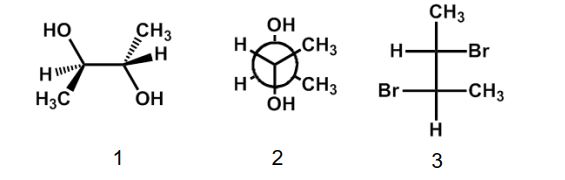
A) 1
B) 2
C) 3
D) 1 and 3
E) none of the above
Structures 1 and 3 are meso and must have R and S asymmetric centers. Structure 2 has no plane of symmetry.

A) 1
B) 2
C) 3
D) 1 and 3
E) none of the above
Structures 1 and 3 are meso and must have R and S asymmetric centers. Structure 2 has no plane of symmetry.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
What is the maximum number of stereoisomers possible for nootkatone (grapefruit oil) shown below?
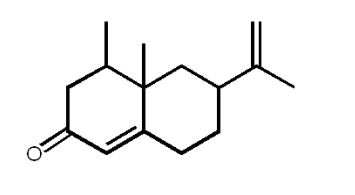
A) 2
B) 3
C) 4
D) 8
E) 16

A) 2
B) 3
C) 4
D) 8
E) 16

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following structures is a meso compound?
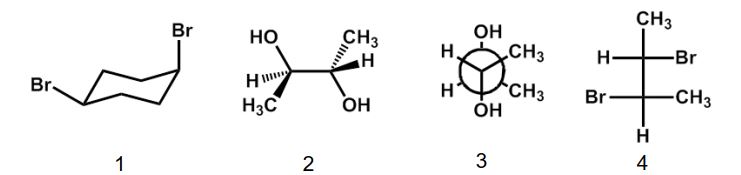
A) 1
B) 2
C) 3
D) 4
E) 2 and 4

A) 1
B) 2
C) 3
D) 4
E) 2 and 4

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
What is the relationship between the two compounds shown below?
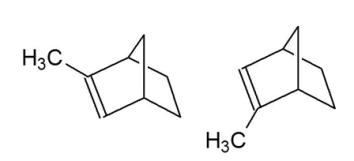
A) enantiomers
B) diastereomers
C) constitutional isomers
D) geometric isomers
E) identical compounds

A) enantiomers
B) diastereomers
C) constitutional isomers
D) geometric isomers
E) identical compounds

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
Using Cahn-Ingold-Prelog rules, which set of answers is correctly organized from highest to lowest priority?
A) ?CH2NH2 > ?CN > -COOH > ?NH2
B) ?COOH > ?CN > ?CH2NH2 > ?NH2
C) ?NH2 > ?CN > ?CH2NH2 > ?COOH
D) ?NH2 > ?COOH > ?CN > ?CH2NH2
E) ?CN > ?NH2 > ?CH2NH2 > ?COOH
Relative priorities are based on atomic number and the rules for multiple bonds (?C N is greater than ?CH2NH2).
N is greater than ?CH2NH2).
A) ?CH2NH2 > ?CN > -COOH > ?NH2
B) ?COOH > ?CN > ?CH2NH2 > ?NH2
C) ?NH2 > ?CN > ?CH2NH2 > ?COOH
D) ?NH2 > ?COOH > ?CN > ?CH2NH2
E) ?CN > ?NH2 > ?CH2NH2 > ?COOH
Relative priorities are based on atomic number and the rules for multiple bonds (?C
 N is greater than ?CH2NH2).
N is greater than ?CH2NH2).
Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck


