Deck 22: Catalysis in Organic Reactions and in Enzymatic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 22: Catalysis in Organic Reactions and in Enzymatic Reactions
1
Which of the following are common types of catalysts used in organic reactions?
A) acid catalysts
B) base catalysts
C) nucleophilic catalysts
D) metal ion catalysts
E) all of the above
A) acid catalysts
B) base catalysts
C) nucleophilic catalysts
D) metal ion catalysts
E) all of the above
all of the above
2
Which of the following is an intermediate in the acid-catalyzed hydrolysis of an ester?
A)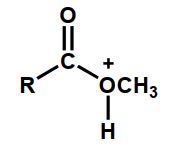
B)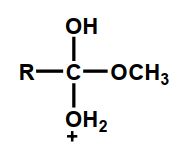
C)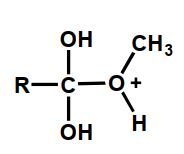
D) Intermediates B and C
E) Intermediates A, B, and C
A)

B)

C)

D) Intermediates B and C
E) Intermediates A, B, and C
Intermediates B and C
3
Which of the following is not true about nucleophilic catalysis?
A) Nucleophilic catalysts form new covalent bonds with the reactants.
B) Nucleophilic catalysis will not involve positively charged intermediates.
C) Nucleophilic catalysis is also called covalent catalysis.
D) A nucleophilic catalyst increases the reaction rate by completely changing the mechanism of the reaction.
E) Nucleophilic catalysts are often good leaving groups.
A) Nucleophilic catalysts form new covalent bonds with the reactants.
B) Nucleophilic catalysis will not involve positively charged intermediates.
C) Nucleophilic catalysis is also called covalent catalysis.
D) A nucleophilic catalyst increases the reaction rate by completely changing the mechanism of the reaction.
E) Nucleophilic catalysts are often good leaving groups.
Nucleophilic catalysis will not involve positively charged intermediates.
4
Which of the following is not true about metal-ion catalysis?
A) It can increase the rate of a reaction by removing a proton from the reactant.
B) It can make a reaction center more susceptible to receiving electrons.
C) It can make a leaving group a weaker base and therefore a better leaving group.
D) It can increase the rate of a hydrolysis reaction by increasing the nucleophilicity of water.
A) It can increase the rate of a reaction by removing a proton from the reactant.
B) It can make a reaction center more susceptible to receiving electrons.
C) It can make a leaving group a weaker base and therefore a better leaving group.
D) It can increase the rate of a hydrolysis reaction by increasing the nucleophilicity of water.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following factors is important in determining the rate of intramolecular reactions?
A) number of collisions per unit time
B) fraction of molecules with sufficient energy
C) fraction of molecules with proper orientation
D) favorable conformation for reaction
E) all of the above
A) number of collisions per unit time
B) fraction of molecules with sufficient energy
C) fraction of molecules with proper orientation
D) favorable conformation for reaction
E) all of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
What type of intramolecular catalysis is occurring in the following reaction? 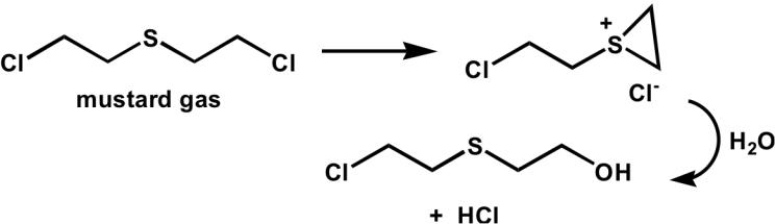
A) acid
B) base
C) nucleophilic
D) metal ion
E) none of the above

A) acid
B) base
C) nucleophilic
D) metal ion
E) none of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
Which factor contributes to the catalytic activity of enzymes?
A) Reacting groups are brought together at the active site in the proper orientation.
B) Amino acid side chains serve as catalysts.
C) Metal ions in the active site act as catalysts.
D) Amino acid side chains stabilize transition states and intermediates.
E) all of the above
A) Reacting groups are brought together at the active site in the proper orientation.
B) Amino acid side chains serve as catalysts.
C) Metal ions in the active site act as catalysts.
D) Amino acid side chains stabilize transition states and intermediates.
E) all of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about enzymatic catalysis is not true?
A) Enzymes are able to provide different solvent environments at the active site.
B) Enzymes allow one group to exist in its acidic form while a nearby group can exist in its basic form.
C) Solvent pH is unimportant in enzymatic reactions.
D) Enzyme activity depends on the reaction mixture pH.
E) A pH-rate profile plots enzyme activity versus pH.
A) Enzymes are able to provide different solvent environments at the active site.
B) Enzymes allow one group to exist in its acidic form while a nearby group can exist in its basic form.
C) Solvent pH is unimportant in enzymatic reactions.
D) Enzyme activity depends on the reaction mixture pH.
E) A pH-rate profile plots enzyme activity versus pH.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about trypsin, chymotrypsin, and elastase are true?
A) They are serine proteases.
B) They are endopeptidases.
C) Serine is part of both tetrahedral intermediates in the enzymatic mechanism.
D) Acid, base, and nucleophilic catalysis are utilized in the overall mechanism for these proteases.
E) All of the above are true.
A) They are serine proteases.
B) They are endopeptidases.
C) Serine is part of both tetrahedral intermediates in the enzymatic mechanism.
D) Acid, base, and nucleophilic catalysis are utilized in the overall mechanism for these proteases.
E) All of the above are true.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about enzyme-catalyzed isomerization of glucose-6-phosphate to fructose-6-phosphate is not true?
A) The mechanism is totally different from the laboratory version of the enediol rearrangement.
B) The first step is acid- and base-catalyzed ring opening.
C) The second step is a base-catalyzed enediol rearrangement.
D) The third step is acid- and base-catalyzed conversion of the enediol to a ketone.
E) The fourth step is acid- and base-catalyzed ring closure.
A) The mechanism is totally different from the laboratory version of the enediol rearrangement.
B) The first step is acid- and base-catalyzed ring opening.
C) The second step is a base-catalyzed enediol rearrangement.
D) The third step is acid- and base-catalyzed conversion of the enediol to a ketone.
E) The fourth step is acid- and base-catalyzed ring closure.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


