Deck 17: Reactions at the Α-Carbon
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/9
Play
Full screen (f)
Deck 17: Reactions at the Α-Carbon
1
Which is the correct order of decreasing acidity (increasing pKa)?
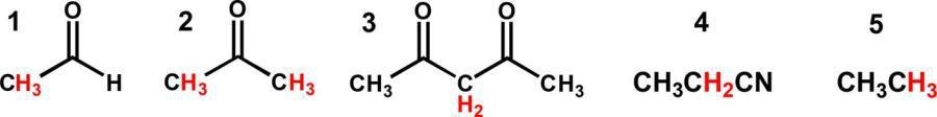
A) 4>3>2>1>5
B) 3>2>1>4>5
C) 5>4>2>1>3
D) 1>2>3>4>5
E) 3>1>2>4>5

A) 4>3>2>1>5
B) 3>2>1>4>5
C) 5>4>2>1>3
D) 1>2>3>4>5
E) 3>1>2>4>5
3>1>2>4>5
2
Which method cannot be used to form an enolate ion from a ketone?
A) KOH, ethanol
B) NaOCH2CH3/ethanol
C) LDA
D) CH3CO2Na
E) NaNH2
A) KOH, ethanol
B) NaOCH2CH3/ethanol
C) LDA
D) CH3CO2Na
E) NaNH2
CH3CO2Na
3
Alpha halogenation of a ketone can be accomplished using____________.
A) Br2, acid catalyst
B) Br2, base catalyst
C) 1. LDA 2. Br2
D) NaBr, H2O, heat
E) all except D
A) Br2, acid catalyst
B) Br2, base catalyst
C) 1. LDA 2. Br2
D) NaBr, H2O, heat
E) all except D
all except D
4
Which compound is the starting material for the following reaction? 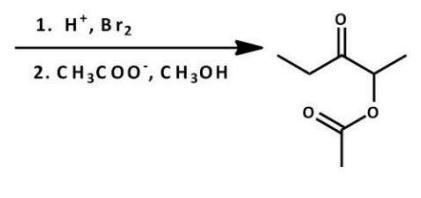
A)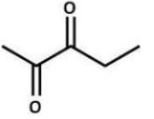
B)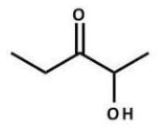
C)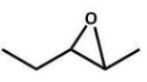
D)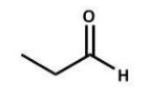
E)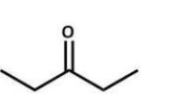

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
5
Which set of reagents can be used to synthesize the following compound? 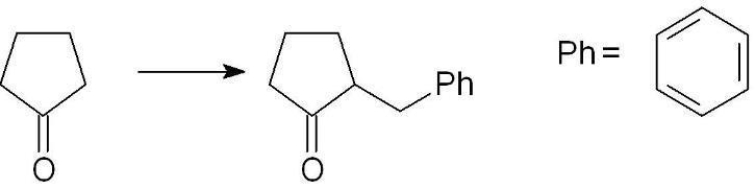
A) 1. CH3NH2/H+ 2. C6H5-Br
B) 1. NaOH 2. C6H5-CH2OTs
C) 1. NaH 2. CH3CH2Br
D) 1. LDA 2. C6H5-CH2Br
E) 1. CH3MgBr 2. C6H5-CH2Br

A) 1. CH3NH2/H+ 2. C6H5-Br
B) 1. NaOH 2. C6H5-CH2OTs
C) 1. NaH 2. CH3CH2Br
D) 1. LDA 2. C6H5-CH2Br
E) 1. CH3MgBr 2. C6H5-CH2Br

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
6
A Michael reaction ____________.
A) forms a 1,5-dicarbonyl compound
B) forms a beta-hydroxyaldehyde or beta-hydroxyketone
C) forms a cyclic beta-keto ester
D) forms a methyl ketone with three more carbons than the alkyl halide
E) forms a carboxylic acid with two more carbons than the alkyl halide
A) forms a 1,5-dicarbonyl compound
B) forms a beta-hydroxyaldehyde or beta-hydroxyketone
C) forms a cyclic beta-keto ester
D) forms a methyl ketone with three more carbons than the alkyl halide
E) forms a carboxylic acid with two more carbons than the alkyl halide

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
7
What type of reaction was used to synthesize the following compound? 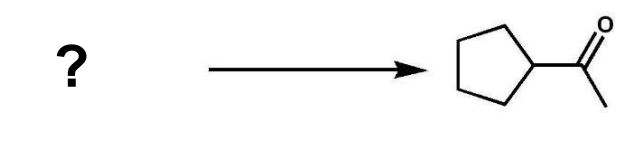
A) aldol condensation
B) acetoacetic ester synthesis
C)Claisen condensation
D) malonic ester synthesis
E) Robinson annulation

A) aldol condensation
B) acetoacetic ester synthesis
C)Claisen condensation
D) malonic ester synthesis
E) Robinson annulation

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
8
What type of reaction was most likely used to prepare the following compound?
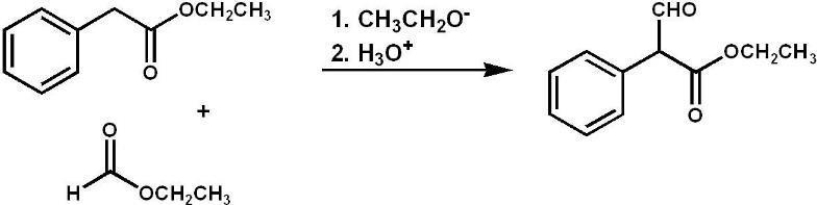
A) aldol condensation
B) acetoacetic ester synthesis
C) mixed Claisen condensation
D) malonic ester synthesis
E) Robinson annulation

A) aldol condensation
B) acetoacetic ester synthesis
C) mixed Claisen condensation
D) malonic ester synthesis
E) Robinson annulation

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
9
What is the major product of the following reaction? 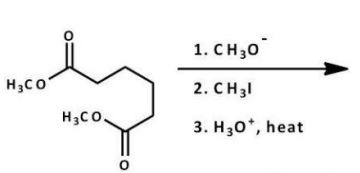
A)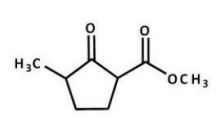
B)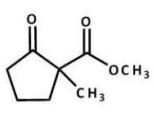
C)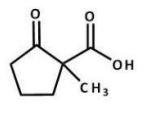
D)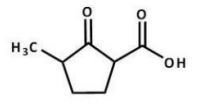
E)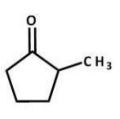

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck


