Deck 16: Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 16: Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid Derivatives
1
What is the systematic name of the following compound?
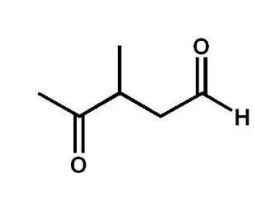
A) 3-methyl-4-oxopentanal
B) 3-methyl-2-oxopentanal
C) 3-methyl-2-oxo-5-pentanal
D) 3-methyl-5-oxo-2-pentanone
E) 3-methylpentan-5-one-1-al

A) 3-methyl-4-oxopentanal
B) 3-methyl-2-oxopentanal
C) 3-methyl-2-oxo-5-pentanal
D) 3-methyl-5-oxo-2-pentanone
E) 3-methylpentan-5-one-1-al
3-methyl-4-oxopentanal
2
What is the correct order of decreasing reactivity toward nucleophilic addition?
1) Acyl halide
2) Aldehyde
3) Carboxylate ion
4) Carboxylic acid
5) Ketone
A) 1>2>3>4>5
B) 1>2>5>4>3
C) 1>5>2>4>3
D) 2>5>1>3>4
E) 5>2>1>3>4
1) Acyl halide
2) Aldehyde
3) Carboxylate ion
4) Carboxylic acid
5) Ketone
A) 1>2>3>4>5
B) 1>2>5>4>3
C) 1>5>2>4>3
D) 2>5>1>3>4
E) 5>2>1>3>4
1>2>5>4>3
3
Which pair of reagents will form the given product? 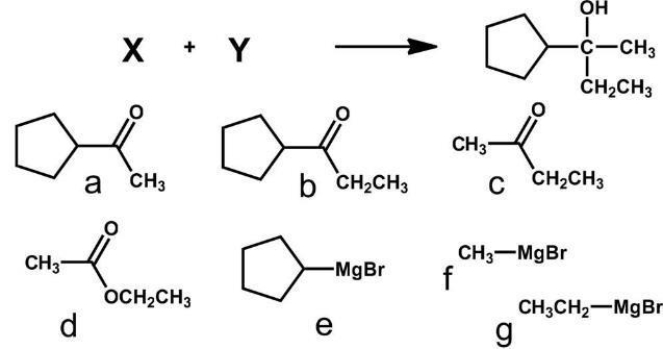
A) a + g
B) b + f
C) c + e
D) d + f
E) all except D

A) a + g
B) b + f
C) c + e
D) d + f
E) all except D
all except D
4
Which reagent(s) will form the given product? 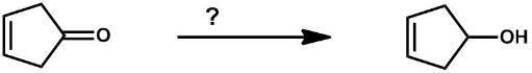
A) NaBH4
B) LiAlH4
C) Raney Ni/H2
D) H2, Pd/C
E) A and B

A) NaBH4
B) LiAlH4
C) Raney Ni/H2
D) H2, Pd/C
E) A and B

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
What is the product of the following reaction? 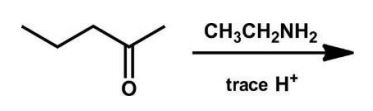
A) an amine
B) a carbinolamine
C) an enamine
D) an imine
E) no reaction

A) an amine
B) a carbinolamine
C) an enamine
D) an imine
E) no reaction

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
Which reagents will form the given product?
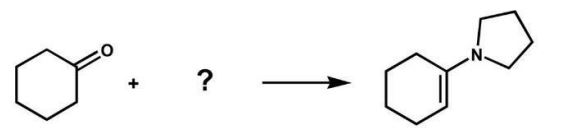
A)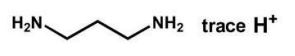
B)
C)
D)
E)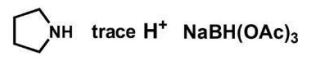

A)

B)

C)

D)

E)
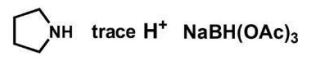

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
The role of an acid catalyst in the formation of an acetal is to____________.
A) protonate the C?O, making it a better electrophile
B) protonate the C?O, making it more reactive toward weak nucleophiles such as alcohols
C) protonate the hemiacetal, which facilitates loss of water
D) do all of the above
A) protonate the C?O, making it a better electrophile
B) protonate the C?O, making it more reactive toward weak nucleophiles such as alcohols
C) protonate the hemiacetal, which facilitates loss of water
D) do all of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
What is the product of the following reaction? 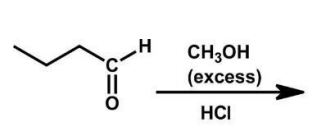
A)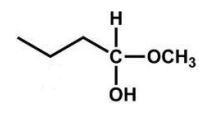
B)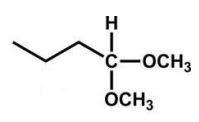
C)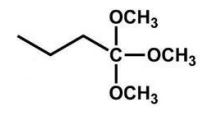
D)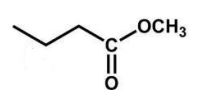
E) No reaction

A)

B)

C)

D)

E) No reaction

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following alkyl halides cannot be used to form a phosphonium ylide?
A)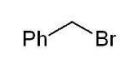
B)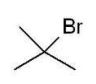
C)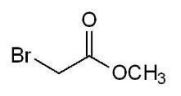
D)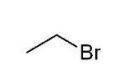
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
What is the major product of the following reaction?
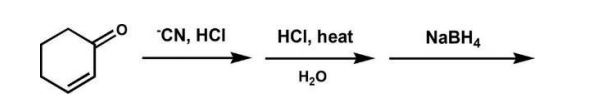
A)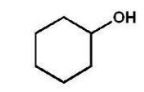
B)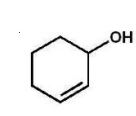
C)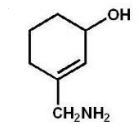
D)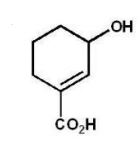
E)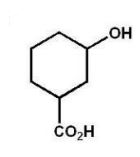

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
What is the major product of the following reaction? 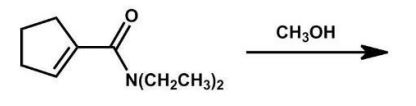
A)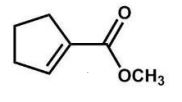
B)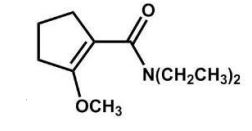
C)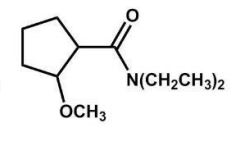
D)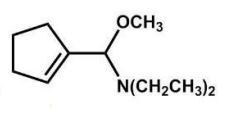
E)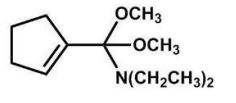

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck


