Deck 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/12
Play
Full screen (f)
Deck 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives
1
What is the name of the compound shown here?
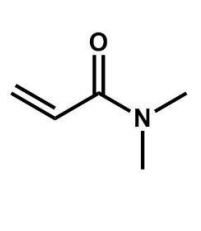
A) N-methyl-N-methylpropanamide
B) N,N-dimethylaminoacrylate
C) N,N-dimethylpropanamine
D) N,N-dimethylpropanamide
E) N,N-dimethylpropenamide

A) N-methyl-N-methylpropanamide
B) N,N-dimethylaminoacrylate
C) N,N-dimethylpropanamine
D) N,N-dimethylpropanamide
E) N,N-dimethylpropenamide
N,N-dimethylpropenamide
2
What is the name of the compound shown here?
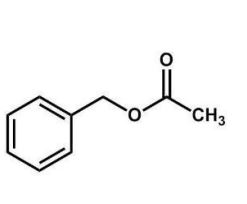
A) acetyl benzene
B) benzene acetic ester
C) benzyl acetate
D) methyl benzoate
E) phenyl acetate

A) acetyl benzene
B) benzene acetic ester
C) benzyl acetate
D) methyl benzoate
E) phenyl acetate
benzyl acetate
3
Which of the following statements is not true about the reactions of carboxylic acid derivatives?
A) Carboxylic acid derivatives form a tetrahedral intermediate.
B) The weaker base will be the leaving group.
C) Less reactive derivatives can be converted directly into more reactive derivatives.
D) For a reaction to occur, the added group must be a stronger base than the group attached to the acyl group.
E) The weakest bond in the reaction is the pi bond, so it breaks first.
A) Carboxylic acid derivatives form a tetrahedral intermediate.
B) The weaker base will be the leaving group.
C) Less reactive derivatives can be converted directly into more reactive derivatives.
D) For a reaction to occur, the added group must be a stronger base than the group attached to the acyl group.
E) The weakest bond in the reaction is the pi bond, so it breaks first.
Less reactive derivatives can be converted directly into more reactive derivatives.
4
Which carboxylic acid derivative is most readily hydrolyzed by aqueous base?
A)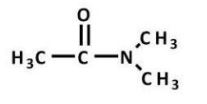
B)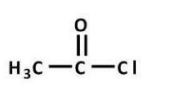
C)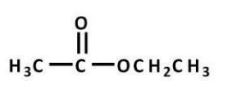
D)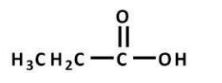
E) Carboxylic acid derivatives hydrolyze by the same mechanism and at the same approximate rate
A)

B)
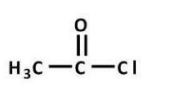
C)
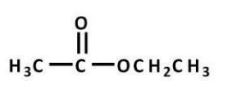
D)
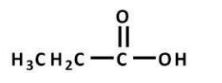
E) Carboxylic acid derivatives hydrolyze by the same mechanism and at the same approximate rate

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
5
In nucleophilic acyl substitution reactions under basic conditions,____________.
A) protonation of the carbonyl group is followed by nucleophilic attack
B) loss of the leaving group is followed by formation of an acylium ion
C) an SN2 reaction occurs
D) the nucleophile must be a weaker base than the leaving group
E) nucleophilic addition to the carbonyl is followed by loss of a leaving group
A) protonation of the carbonyl group is followed by nucleophilic attack
B) loss of the leaving group is followed by formation of an acylium ion
C) an SN2 reaction occurs
D) the nucleophile must be a weaker base than the leaving group
E) nucleophilic addition to the carbonyl is followed by loss of a leaving group

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following reactions will proceed to the right? 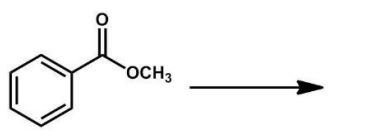
A) excess H2O, HCl, heat
B) phenol, HCl
C) CH3NH2, heat
D) NaNH2
E) all except B

A) excess H2O, HCl, heat
B) phenol, HCl
C) CH3NH2, heat
D) NaNH2
E) all except B

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following carboxylic acid derivatives could be used in this reaction?
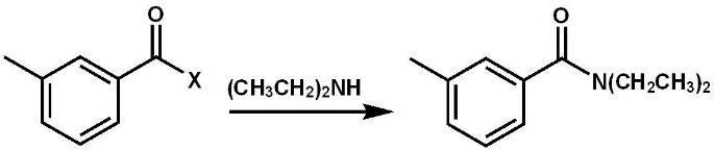 For X =
For X =
A) ?Br
B) ?Cl
C) ?SCH3
D) ?OCH3
E) all of the above
 For X =
For X =A) ?Br
B) ?Cl
C) ?SCH3
D) ?OCH3
E) all of the above

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
8
What are the expected products of the following reaction? 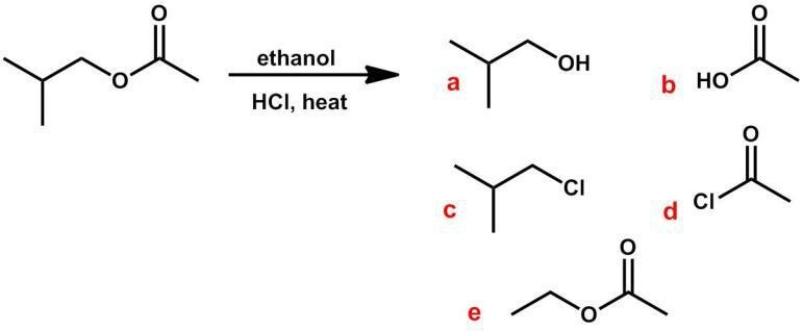
A) a, b
B) a, d
C) c, b
D) c, d
E) a, e

A) a, b
B) a, d
C) c, b
D) c, d
E) a, e

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
9
Upon aqueous hydrolysis of 18O-labeled ethyl acetate, which oxygen(s) will be labeled in the final reaction mixture after mild acidification?
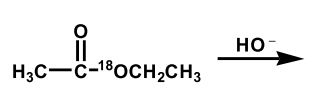
A)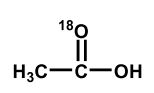
B)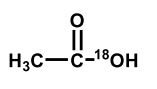
C)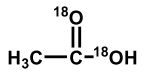
D)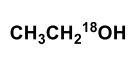
E)

A)

B)

C)

D)
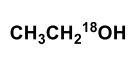
E)


Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
10
Which compound is the product of the following reaction sequence? 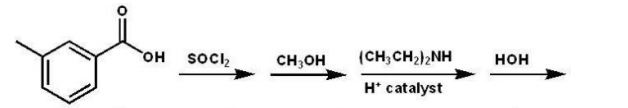
A)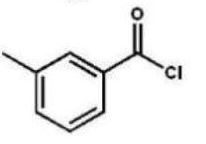
B)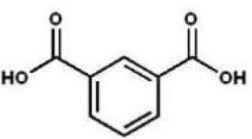
C)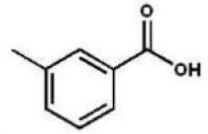
D)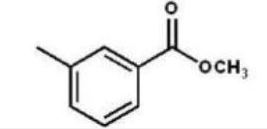
E)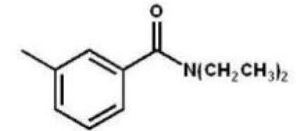

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
11
Which set of reagents will produce the indicated product? 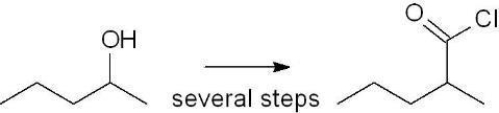
A) 1. H2CrO4 2. SOCl2/pyridine 3. H2O
B) 1. TsCl/pyridine 2. NaCN 3. H3O+/? 4. SOCl2/pyridine
C) 1. Acetic anhydride 2. SOCl2/pyridine
D) 1. Acetic acid/H3O+ 2. PCl3/pyridine
E) 1. Ethyl acetate/H3O+ 2. NaCl

A) 1. H2CrO4 2. SOCl2/pyridine 3. H2O
B) 1. TsCl/pyridine 2. NaCN 3. H3O+/? 4. SOCl2/pyridine
C) 1. Acetic anhydride 2. SOCl2/pyridine
D) 1. Acetic acid/H3O+ 2. PCl3/pyridine
E) 1. Ethyl acetate/H3O+ 2. NaCl

Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following dicarboxylic acids has the most similar pKa1 and pKa2 values?
A)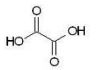
B)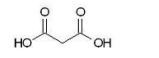
C)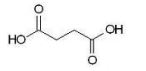
D)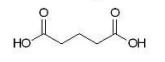
E)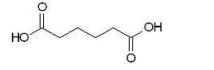
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 12 flashcards in this deck.
Unlock Deck
k this deck


