Deck 12: Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/156
Play
Full screen (f)
Deck 12: Chemical Bonding
1
What person is known as the "father of chemistry" for his many contributions,including the law of conservation of mass?
A) Boyle
B) Lavoisier
C) Mendeleev
D) Dalton
A) Boyle
B) Lavoisier
C) Mendeleev
D) Dalton
B
2
The percentage by mass of carbon in diamond is about
A) 25%.
B) 75%.
C) 100%.
D) 20%.
A) 25%.
B) 75%.
C) 100%.
D) 20%.
C
3
A substance of unknown composition is heated in a sealed container.As a result,the mass of the container and its contents
A) increases.
B) decreases.
C) remains the same.
D) Any of these could occur.
A) increases.
B) decreases.
C) remains the same.
D) Any of these could occur.
C
4
The force responsible for chemical bonding is
A) nuclear.
B) electromagnetic.
C) none of these.
D) gravitational.
A) nuclear.
B) electromagnetic.
C) none of these.
D) gravitational.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
5
The percentage composition by mass of water is approximately
A) 2% H and 1% O.
B) 11% H and 89% O.
C) 1% H and 99% O.
D) 2% H and 16% O.
A) 2% H and 1% O.
B) 11% H and 89% O.
C) 1% H and 99% O.
D) 2% H and 16% O.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
6
An element that tends to gain electrons during chemical reactions is probably
A) a semimetal.
B) a nonmetal.
C) an actinide.
D) a metal.
A) a semimetal.
B) a nonmetal.
C) an actinide.
D) a metal.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
7
What is the formula mass of ammonium phosphide,(NH4)3P?
A) 85.0 u
B) 49.0 u
C) 48.0 u
D) 57.0 u
A) 85.0 u
B) 49.0 u
C) 48.0 u
D) 57.0 u

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
8
The formula mass of calcium hydroxide,Ca(OH)2,is
A) 74.1 u.
B) 38.1 u.
C) 57.1 u.
D) 58.1 u.
A) 74.1 u.
B) 38.1 u.
C) 57.1 u.
D) 58.1 u.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
9
The percentage by mass of hydrogen in methane,CH4,is about
A) 25%.
B) 75%.
C) 80%.
D) 20%.
A) 25%.
B) 75%.
C) 80%.
D) 20%.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
10
The percentage by mass of carbon
A) would be the same in CO as in CO2.
B) would be greater in CO than in CO2.
C) would be less in CO than in CO2.
D) cannot be determined without additional information.
A) would be the same in CO as in CO2.
B) would be greater in CO than in CO2.
C) would be less in CO than in CO2.
D) cannot be determined without additional information.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
11
The percentage by mass of chlorine
A) would be the same in FeCl3 as in FeCl2.
B) would be greater in FeCl3 than in FeCl2.
C) would be less in FeCl3 than in FeCl2.
D) cannot be determined without additional information.
A) would be the same in FeCl3 as in FeCl2.
B) would be greater in FeCl3 than in FeCl2.
C) would be less in FeCl3 than in FeCl2.
D) cannot be determined without additional information.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
12
Another name for solid carbon dioxide is
A) heavy water.
B) Epsom salt.
C) dry ice.
D) quicksilver.
A) heavy water.
B) Epsom salt.
C) dry ice.
D) quicksilver.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
13
A substance of unknown composition is heated in an open container.As a result,the mass of the container and its contents
A) decreases.
B) remains the same.
C) increases.
D) Any of these could occur.
A) decreases.
B) remains the same.
C) increases.
D) Any of these could occur.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
14
The law of definite proportions states that different samples of a pure compound always contain the same elements in the same proportion by
A) mass.
B) atoms.
C) atomic number.
D) volume.
A) mass.
B) atoms.
C) atomic number.
D) volume.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
15
The percentage by mass of carbon in methane,CH4,is about
A) 25%.
B) 75%.
C) 80%.
D) 20%.
A) 25%.
B) 75%.
C) 80%.
D) 20%.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
16
The percentage composition by mass of nitrogen dioxide is approximately
A) 43% N and 57% O.
B) 33% N and 67% O.
C) 30% N and 70% O.
D) 47% N and 53% O.
A) 43% N and 57% O.
B) 33% N and 67% O.
C) 30% N and 70% O.
D) 47% N and 53% O.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
17
A compound consists of only magnesium,carbon,and oxygen.If the percentage by mass of Mg is 28.8% and that of C is 14.2%,what is the percentage by mass of O?
A) 16.0%
B) 57.0%
C) 43.0%
D) 22.5%
E) None of the above
A) 16.0%
B) 57.0%
C) 43.0%
D) 22.5%
E) None of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
18
The formula mass of sulfuric acid,H2SO4,is
A) 98 u.
B) 100 u.
C) 66 u.
D) 7 u.
A) 98 u.
B) 100 u.
C) 66 u.
D) 7 u.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
19
The ratio by mass of carbon to hydrogen in 100 g of a certain compound is 6 to 1.The ratio in 200 g of the same compound will be
A) 24 to 1.
B) 6 to 1.
C) 12 to 1.
D) none of these.
A) 24 to 1.
B) 6 to 1.
C) 12 to 1.
D) none of these.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
20
The formula mass of water,H2O,is
A) 18 u.
B) 9 u.
C) 10 u.
D) 17 u.
A) 18 u.
B) 9 u.
C) 10 u.
D) 17 u.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
21
What electric charge is characteristic for an ion of Cl?
A) 7+
B) 0
C) 1-
D) 1+
A) 7+
B) 0
C) 1-
D) 1+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
22
Suppose 25 g of reactant A is mixed with 45 g of reactant B.After a chemical reaction takes place between A and B,which statement is correct?
A) The total mass of products plus any unreacted reactants will be 70 g.
B) The total mass of products plus any unreacted reactants will be less than 70 g.
C) The total mass of products plus any unreacted reactants will be more than 70 g.
D) The total mass of products will be 70 g.
A) The total mass of products plus any unreacted reactants will be 70 g.
B) The total mass of products plus any unreacted reactants will be less than 70 g.
C) The total mass of products plus any unreacted reactants will be more than 70 g.
D) The total mass of products will be 70 g.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
23
What electric charge is characteristic for an ion of F?
A) 7+
B) 0
C) 1-
D) 1+
A) 7+
B) 0
C) 1-
D) 1+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
24
A likely ratio of the masses of oxygen in two different compounds of tin and oxygen,both of which contain the same mass of tin,is
A) 1.42 to 1.
B) 3.11 to 1.
C) 3.77 to 1.
D) 2 to 1.
A) 1.42 to 1.
B) 3.11 to 1.
C) 3.77 to 1.
D) 2 to 1.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
25
Element A,mass 5.00 g,reacts exactly with 2.00 g of element B to form 7.00 g of compound AB.If 10.00 g of element A are reacted with 2.00 g of element B to form the same compound,what mass of compound AB will be formed?
A) 7.00 g
B) 5.00 g
C) 17.00 g
D) 12.00 g
A) 7.00 g
B) 5.00 g
C) 17.00 g
D) 12.00 g

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
26
When potassium and sulfur react to form potassium sulfide,an ionic compound,
A) each sulfur atom loses two electrons.
B) each sulfur atom loses one electron.
C) each potassium atom loses one electron.
D) each potassium atom loses two electrons.
A) each sulfur atom loses two electrons.
B) each sulfur atom loses one electron.
C) each potassium atom loses one electron.
D) each potassium atom loses two electrons.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
27
What electric charge is characteristic for an ion of O?
A) 6+
B) 0
C) 2-
D) 2+
A) 6+
B) 0
C) 2-
D) 2+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
28
What electric charge is characteristic for an ion of S?
A) 6+
B) 0
C) 2-
D) 2+
A) 6+
B) 0
C) 2-
D) 2+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
29
When sodium and chlorine react to form sodium chloride,an ionic compound,
A) each sodium atom loses two electrons.
B) each sodium atom loses one electron.
C) each chlorine atom loses one electron.
D) each chlorine atom loses two electrons.
A) each sodium atom loses two electrons.
B) each sodium atom loses one electron.
C) each chlorine atom loses one electron.
D) each chlorine atom loses two electrons.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
30
When sodium and chlorine react to form sodium chloride,an ionic compound,
A) each sodium atom gains two electrons.
B) each sodium atom gains one electron.
C) each chlorine atom gains one electron.
D) each chlorine atom gains two electrons.
A) each sodium atom gains two electrons.
B) each sodium atom gains one electron.
C) each chlorine atom gains one electron.
D) each chlorine atom gains two electrons.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
31
Suppose 39 g of reactant A is mixed with 40 g of reactant B.After a chemical reaction takes place between A and B,which statement is correct?
A) The total mass of products plus any unreacted reactants will be 79 g.
B) The total mass of products plus any unreacted reactants will be less than 79 g.
C) The total mass of products plus any unreacted reactants will be more than 79 g.
D) The total mass of products will be 79 g.
A) The total mass of products plus any unreacted reactants will be 79 g.
B) The total mass of products plus any unreacted reactants will be less than 79 g.
C) The total mass of products plus any unreacted reactants will be more than 79 g.
D) The total mass of products will be 79 g.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following was not a part of Dalton's atomic theory?
A) Each element is composed of atoms, which are identical for that element.
B) The reactivity of elements involves changes in their electron configuration.
C) Chemical combination is the bonding of a definite number of atoms of each of the combining elements.
D) No atoms are gained, lost, or changed in identity during a chemical reaction.
A) Each element is composed of atoms, which are identical for that element.
B) The reactivity of elements involves changes in their electron configuration.
C) Chemical combination is the bonding of a definite number of atoms of each of the combining elements.
D) No atoms are gained, lost, or changed in identity during a chemical reaction.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
33
Element A,mass 9.00 g,reacts exactly with 5.00 g of element B to form 14.00 g of compound AB.If 36.00 g of element A is reacted with 41.00 g of element B to form the same compound,what mass of compound AB will be formed?
A) 9.00 g
B) 56.00 g
C) 9.00 g
D) 30.00 g
A) 9.00 g
B) 56.00 g
C) 9.00 g
D) 30.00 g

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
34
For a compound composed of ions,the smallest combination of ions that gives the formula of the compound is called a
A) molecule.
B) polyionic atom.
C) polyatomic ion.
D) formula unit.
A) molecule.
B) polyionic atom.
C) polyatomic ion.
D) formula unit.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
35
A general principle of ionic compound formation is that the total ionic charge in the formula unit must be
A) 0.
B) no greater than that of the metal ion.
C) between +4 and -4.
D) no less than that of the nonmetal ion.
A) 0.
B) no greater than that of the metal ion.
C) between +4 and -4.
D) no less than that of the nonmetal ion.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
36
The atomic theory was revived and put on a solid experimental foundation in about 1803 by
A) Proust.
B) Dalton.
C) Boyle.
D) Lavoisier.
A) Proust.
B) Dalton.
C) Boyle.
D) Lavoisier.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
37
What is the formula of a compound formed from the ions M1+ and X3-?
A) M3X
B) MX3
C) MX
D) None of these
A) M3X
B) MX3
C) MX
D) None of these

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
38
Element A,mass 10.00 g,reacts exactly with 2.00 g of element B to form 12.00 g of compound AB.If 5.00 g of element A is reacted with 2.00 g of element B to form the same compound,what mass of compound AB will be formed?
A) 7.00 g
B) 6.00 g
C) 17.00 g
D) 12.00 g
A) 7.00 g
B) 6.00 g
C) 17.00 g
D) 12.00 g

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
39
A compound consists of only magnesium,carbon,and oxygen.If the percentage by mass of Mg is 63.9% and that of C is 12.2%,what is the percentage by mass of O?
A) 76.1%
B) 23.9%
C) 51.7%
D) 70.9%
E) None of the above
A) 76.1%
B) 23.9%
C) 51.7%
D) 70.9%
E) None of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
40
The octet rule states that the formula for a compound is derived by
A) using a minimum of 8 atoms.
B) using exactly 8 bonds in the formula unit.
C) having all atoms or ions get noble gas electron configurations.
D) having a net charge of magnitude 8.
A) using a minimum of 8 atoms.
B) using exactly 8 bonds in the formula unit.
C) having all atoms or ions get noble gas electron configurations.
D) having a net charge of magnitude 8.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
41
What is the formula of a compound formed from the ions M3+ and X2-?
A) M2X
B) MX
C) M2X3
D) MX2
A) M2X
B) MX
C) M2X3
D) MX2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
42
What is the formula of a compound formed from the ions M3+ and X3-?
A) M2X
B) MX
C) M2X3
D) MX2
A) M2X
B) MX
C) M2X3
D) MX2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formula of the compound formed from the ions of calcium and bromine?
A) CaBr3
B) CaBr
C) CaBr2
D) Ca2Br3
E) None of these
A) CaBr3
B) CaBr
C) CaBr2
D) Ca2Br3
E) None of these

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
44
What is the formula of a compound formed from the ions M2+ and X3-?
A) MX
B) M3X2
C) M2X3
D) M6X6
A) MX
B) M3X2
C) M2X3
D) M6X6

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
45
The Lewis symbol would be characteristic of which group 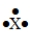 ?
?
A) 2A
B) 4A
C) 3A
D) 1A
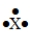 ?
?A) 2A
B) 4A
C) 3A
D) 1A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
46
The Lewis symbol would be characteristic of which group 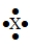 ?
?
A) 2A
B) 4A
C) 6A
D) 1A
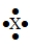 ?
?A) 2A
B) 4A
C) 6A
D) 1A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
47
The Lewis symbol would be characteristic of which group 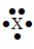 ?
?
A) 2A
B) 4A
C) 3A
D) 5A
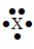 ?
?A) 2A
B) 4A
C) 3A
D) 5A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
48
What is the formula of a compound formed from the ions M3+ and X1-?
A) M3X
B) MX
C) M2X3
D) MX3
A) M3X
B) MX
C) M2X3
D) MX3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
49
Combinations of atoms from the far right side of the periodic table with atoms from the far left side usually form ______________ compounds.
A) organic
B) ionic
C) covalent
D) polar
A) organic
B) ionic
C) covalent
D) polar

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
50
What is the formula of the compound formed from the ions of aluminum and oxygen?
A) Al2O3
B) AlO
C) AlO3
D) None of these
A) Al2O3
B) AlO
C) AlO3
D) None of these

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
51
The Lewis symbol would be characteristic of which group 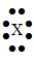 ?
?
A) 6A
B) 8A
C) 3A
D) 7A
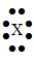 ?
?A) 6A
B) 8A
C) 3A
D) 7A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
52
What is the formula of a compound formed from the ions M1+ and X1-?
A) M2X
B) MX
C) M2X2
D) MX2
A) M2X
B) MX
C) M2X2
D) MX2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
53
The Lewis symbol X• would be characteristic of which group?
A) 2A
B) 4A
C) 6A
D) 1A
A) 2A
B) 4A
C) 6A
D) 1A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
54
The Lewis symbol would be characteristic of which group 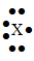 ?
?
A) 6A
B) 1A
C) 3A
D) 7A
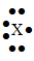 ?
?A) 6A
B) 1A
C) 3A
D) 7A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
55
If atoms unite by transfer of electrons,they form
A) an organic compound.
B) an ionic compound.
C) a polar molecule.
D) a covalent compound.
A) an organic compound.
B) an ionic compound.
C) a polar molecule.
D) a covalent compound.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
56
What is the formula of a compound formed from the ions M2+ and X2-?
A) M2X
B) MX
C) M2X2
D) MX2
A) M2X
B) MX
C) M2X2
D) MX2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
57
The Lewis symbol would be characteristic of which group 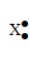 ?
?
A) 6A
B) 8A
C) 3A
D) 7A
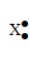 ?
?A) 6A
B) 8A
C) 3A
D) 7A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
58
The Lewis symbol •X• would be characteristic of which group?
A) 2A
B) 4A
C) 6A
D) 1A
A) 2A
B) 4A
C) 6A
D) 1A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
59
If a compound is solid,has a high melting point,conducts electricity in the liquid phase,and dissolves in water,it is most likely to be a(n)______________ compound.
A) intermediate
B) organic
C) covalent
D) ionic
A) intermediate
B) organic
C) covalent
D) ionic

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
60
The Lewis symbol would be characteristic of which group 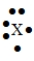 ?
?
A) 6A
B) 2A
C) 3A
D) 5A
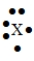 ?
?A) 6A
B) 2A
C) 3A
D) 5A

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
61
If two atoms have five electrons in the valence shell and are covalently bound,the molecule has a ______________ bond.
A) quadruple
B) double
C) triple
D) single
A) quadruple
B) double
C) triple
D) single

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following ions would never form in chemical reactions?
A) F2-
B) Al3+
C) O2-
D) N3-
A) F2-
B) Al3+
C) O2-
D) N3-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
63
Which two elements are most likely to combine with one another to form an ionic compound?
A) B and Cl
B) Ra and Br
C) Mg and Ca
D) S and O
A) B and Cl
B) Ra and Br
C) Mg and Ca
D) S and O

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
64
If two atoms have equal electronegativity,a bond formed between them is generally
A) ionic.
B) nonpolar.
C) very weak.
D) polar.
A) ionic.
B) nonpolar.
C) very weak.
D) polar.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following compounds would be expected to be hard and brittle and to have a high melting point?
A) H2SO4
B) KBr
C) H2C2O4
D) IF7
A) H2SO4
B) KBr
C) H2C2O4
D) IF7

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
66
The sharing of two electrons by unlike atoms generally forms a(n)______________ bond.
A) ionic
B) metallic
C) nonpolar
D) polar covalent
A) ionic
B) metallic
C) nonpolar
D) polar covalent

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
67
What is the correct formula for calcium phosphate?
A) Ca3P2
B) Ca2(PO4)3
C) Ca3(PO4)2
D) Ca2P3
A) Ca3P2
B) Ca2(PO4)3
C) Ca3(PO4)2
D) Ca2P3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
68
When hydrogen,from Group 1A,unites with chlorine,it forms a(n)______________ compound.
A) ionic
B) metallic
C) covalent
D) organic
A) ionic
B) metallic
C) covalent
D) organic

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
69
Except for hydrogen and helium,atoms in chemical combination tend to have ______________ electrons in their outer shell.
A) 8
B) 14
C) 6
D) 2
A) 8
B) 14
C) 6
D) 2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
70
The Lewis symbol of which of the following elements has a single dot on each of the four sides of the element's symbol?
A) Helium
B) Carbon
C) Beryllium
D) None of these
A) Helium
B) Carbon
C) Beryllium
D) None of these

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following elements commonly forms an ion with a 1- charge?
A) Ag
B) N
C) Br
D) Na
A) Ag
B) N
C) Br
D) Na

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
72
The ionic charge of the nitrite ion in Al(NO2)3,aluminum nitrite,must be
A) 3+.
B) 1-.
C) 3-.
D) 1+.
A) 3+.
B) 1-.
C) 3-.
D) 1+.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
73
Two atoms of hydrogen bond with an atom of oxygen to form a water molecule.This bonding is
A) covalent only.
B) polar only.
C) both covalent and polar.
D) none of these.
A) covalent only.
B) polar only.
C) both covalent and polar.
D) none of these.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following ions is isoelectronic with a neon atom?
A) N3-
B) Li+
C) Cl-
D) F2-
A) N3-
B) Li+
C) Cl-
D) F2-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
75
The ionic compound made of Fe3+ and CO32- ions would have the formula
A) Fe3(CO3)2.
B) FeCO3.
C) Fe2(CO3)3.
D) none of these
A) Fe3(CO3)2.
B) FeCO3.
C) Fe2(CO3)3.
D) none of these

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
76
A Group 6A nonmetal would be most likely to form an ionic bond with an element of Group
A) 4A.
B) 8A.
C) 2A.
D) 5A.
A) 4A.
B) 8A.
C) 2A.
D) 5A.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following ions is not isoelectronic with the other three?
A) Cl-
B) K+
C) Br-
D) P3-
A) Cl-
B) K+
C) Br-
D) P3-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
78
When atoms unite by transferring electrons,they should form
A) a polar molecule.
B) a covalent compound.
C) an ionic compound.
D) an organic compound.
A) a polar molecule.
B) a covalent compound.
C) an ionic compound.
D) an organic compound.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
79
A pair of electrons shared by two atoms forms
A) a metallic bond.
B) a double covalent bond.
C) a single covalent bond.
D) an ionic bond.
A) a metallic bond.
B) a double covalent bond.
C) a single covalent bond.
D) an ionic bond.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
80
The anion in the common ionic substance NaHCO3 is
A) CO32-.
B) H+.
C) Na+.
D) HCO3-.
A) CO32-.
B) H+.
C) Na+.
D) HCO3-.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck


