Deck 5: Temperature and Heat
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 5: Temperature and Heat
1
The Celsius degree is
A) the same size as the Fahrenheit degree.
B) larger than the kelvin.
C) the same size as the kelvin.
D) smaller than the Fahrenheit degree.
A) the same size as the Fahrenheit degree.
B) larger than the kelvin.
C) the same size as the kelvin.
D) smaller than the Fahrenheit degree.
C
2
A temperature of -65 Kelvin is equivalent to _____ degrees Fahrenheit.
A) -576.7
B) 208.0
C) -53.9
D) -338.0
A) -576.7
B) 208.0
C) -53.9
D) -338.0
A
3
The amount of heat necessary to change the temperature of 1 kg of a substance 1°C is
A) 1 cal.
B) the specific heat.
C) the heat difference.
D) 1 J.
A) 1 cal.
B) the specific heat.
C) the heat difference.
D) 1 J.
B
4
One kelvin unit is equivalent to
A) 9/5 Fahrenheit degrees.
B) 1 Fahrenheit degree.
C) 2 Celsius degrees.
D) 1.8 Celsius degrees.
A) 9/5 Fahrenheit degrees.
B) 1 Fahrenheit degree.
C) 2 Celsius degrees.
D) 1.8 Celsius degrees.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
In measuring any temperature less than 500 Fahrenheit,which of the following scales will have the highest numeric reading?
A) Fahrenheit
B) Celsius
C) Kelvin
D) None of these
A) Fahrenheit
B) Celsius
C) Kelvin
D) None of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
A temperature of degrees 250 Celsius is equivalent to _____ degrees Fahrenheit.
A) -9.4
B) 482.0
C) 121.1
D) -23.0
A) -9.4
B) 482.0
C) 121.1
D) -23.0

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
Temperature is a relative measure of
A) latent heat.
B) hotness and coldness.
C) internal energy.
D) specific heat.
A) latent heat.
B) hotness and coldness.
C) internal energy.
D) specific heat.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
The heating of a room by a fire in a fireplace is chiefly due to
A) vaporization.
B) radiation.
C) convection.
D) conduction.
A) vaporization.
B) radiation.
C) convection.
D) conduction.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
A digital thermometer can make readings to the tenth of a degree at either the Celsius setting or the Fahrenheit setting.Which reading would be more precise?
A) The Fahrenheit setting, since 1 degree Fahrenheit is smaller than 1 degree Celsius
B) The Fahrenheit setting, since 1 degree Celsius is smaller than 1 degree Fahrenheit
C) The Celsius setting, since 1 degree Fahrenheit is smaller than 1 degree Celsius
D) The Celsius setting, since 1 degree Celsius is smaller than 1 degree Fahrenheit
A) The Fahrenheit setting, since 1 degree Fahrenheit is smaller than 1 degree Celsius
B) The Fahrenheit setting, since 1 degree Celsius is smaller than 1 degree Fahrenheit
C) The Celsius setting, since 1 degree Fahrenheit is smaller than 1 degree Celsius
D) The Celsius setting, since 1 degree Celsius is smaller than 1 degree Fahrenheit

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
The Sun's rays are transmitted to Earth by means of
A) radiation.
B) convection.
C) temperature.
D) conduction.
A) radiation.
B) convection.
C) temperature.
D) conduction.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
A temperature of degrees 5 Fahrenheit is equivalent to _____ Kelvin.
A) 258.2
B) 278.0
C) -15.0
D) -268.0
A) 258.2
B) 278.0
C) -15.0
D) -268.0

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
The SI unit of heat is the
A) calorie.
B) joule.
C) kilocalorie.
D) Btu.
A) calorie.
B) joule.
C) kilocalorie.
D) Btu.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
A temperature of degrees 290 Fahrenheit is equivalent to _____ degrees Celsius.
A) 62.6
B) 554.0
C) 143.3
D) 17.0
A) 62.6
B) 554.0
C) 143.3
D) 17.0

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following temperature scales has the smallest unit interval?
A) Celsius
B) Kelvin
C) Fahrenheit
D) None of these
A) Celsius
B) Kelvin
C) Fahrenheit
D) None of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
A large calorie (Cal)is the amount of heat needed to change the temperature of one ______________ of water by one Celsius degree.
A) dekagram
B) centigram
C) gram
D) kilogram
A) dekagram
B) centigram
C) gram
D) kilogram

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
The average kinetic energy of the molecules in a gas is a measure of
A) volume.
B) density.
C) temperature.
D) heat content.
A) volume.
B) density.
C) temperature.
D) heat content.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
Heat transfer that involves mass movement is
A) temperature.
B) conduction.
C) convection.
D) radiation.
A) temperature.
B) conduction.
C) convection.
D) radiation.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
The units of specific heat are
A) kcal-kg/°C.
B) J/kg-°C.
C) kcal-kg.
D) J/kg.
A) kcal-kg/°C.
B) J/kg-°C.
C) kcal-kg.
D) J/kg.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
A temperature of degrees 260 Celsius is equivalent to _____ Kelvin.
A) 8.6
B) 533.2
C) 126.7
D) -13.0
A) 8.6
B) 533.2
C) 126.7
D) -13.0

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
A temperature of -65 Kelvin is equivalent to _____ degrees Celsius.
A) 219.1
B) 208.0
C) -53.9
D) -338.2
A) 219.1
B) 208.0
C) -53.9
D) -338.2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
Heat transfer from hot or cold liquids in a thermos bottle is prevented by ______________.
A) convection
B) conduction
C) radiation
D) all of these
A) convection
B) conduction
C) radiation
D) all of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
The amount of heat necessary to change 1 kg of a solid into a liquid at the same temperature is called the
A) entropy.
B) melting point.
C) latent heat of vaporization.
D) latent heat of fusion.
A) entropy.
B) melting point.
C) latent heat of vaporization.
D) latent heat of fusion.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
How much heat is required to melt 3.0 kg of ice at 0°C to water at 0°C?
A) 240 kcal
B) 0 kcal
C) 1620 kcal
D) 360 kcal
A) 240 kcal
B) 0 kcal
C) 1620 kcal
D) 360 kcal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
On a winter day the temperature drops from -15°C to -25°C overnight.If a pan sitting outside contains 0.20 kg of ice,how much heat is removed from the ice for this temperature change?
A) 0.1 kcal
B) 1 kcal
C) 4 kcal
D) 0.4 kcal
A) 0.1 kcal
B) 1 kcal
C) 4 kcal
D) 0.4 kcal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following has the lowest thermal conductivity?
A) Glass
B) Iron
C) Wood
D) Styrofoam
A) Glass
B) Iron
C) Wood
D) Styrofoam

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
An ideal gas is confined to a container with an adjustable volume.If the number of molecules and the temperature are held constant,by what factor will the volume change when the pressure is quintupled?
A) 25
B) 0.2
C) 0.04
D) 5
A) 25
B) 0.2
C) 0.04
D) 5

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
A home fireplace loses most of its heat by
A) radiation into the room.
B) convection up the chimney.
C) radiation up the chimney.
D) conduction to the sides of the fireplace.
A) radiation into the room.
B) convection up the chimney.
C) radiation up the chimney.
D) conduction to the sides of the fireplace.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
The amount of heat necessary to change 1 kg of a liquid into a gas at the same temperature is called the
A) entropy.
B) boiling point.
C) latent heat of vaporization.
D) latent heat of fusion.
A) entropy.
B) boiling point.
C) latent heat of vaporization.
D) latent heat of fusion.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
A sample of gas has its number of molecules quadrupled,its Kelvin temperature doubled,and its volume tripled.By what factor has the new pressure changed relative to the original pressure?
A) 2.7
B) 1.7
C) 24.3
D) 24
A) 2.7
B) 1.7
C) 24.3
D) 24

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
A circular hole is drilled into an aluminum sheet.When the sheet is heated,the diameter of the hole will
A) become smaller.
B) become larger.
C) depend on the thickness of the sheet.
D) not be affected.
A) become smaller.
B) become larger.
C) depend on the thickness of the sheet.
D) not be affected.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
The number of molecules in a container is quadrupled and the Kelvin temperature quadrupled.The volume remains unchanged.The new pressure will be how many times greater than the original pressure?
A) 16
B) 1
C) 20
D) 32
A) 16
B) 1
C) 20
D) 32

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
How much heat is necessary to change 30 g of water at 10°C into water at 80°C?
A) 0.21 kcal
B) 2.1 kcal
C) 4.5 kcal
D) 0.51 kcal
A) 0.21 kcal
B) 2.1 kcal
C) 4.5 kcal
D) 0.51 kcal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
Which laboratory observation would most support the kinetic theory's assumption that gas molecules have little attraction for one another?
A) A gas expands when the pressure on it is released.
B) A gas with a lower formula mass diffuses more rapidly than one with a higher formula mass.
C) A gas exerts the same pressure on each part of a container's wall.
D) A gas has mass.
A) A gas expands when the pressure on it is released.
B) A gas with a lower formula mass diffuses more rapidly than one with a higher formula mass.
C) A gas exerts the same pressure on each part of a container's wall.
D) A gas has mass.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
A change of phase takes place at a constant
A) temperature.
B) volume.
C) heat.
D) pressure.
A) temperature.
B) volume.
C) heat.
D) pressure.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
How much heat is necessary to change 10 g of water at 30°C into steam at 100°C?
A) 0.07 kcal
B) 0.7 kcal
C) 6.1 kcal
D) 0.67 kcal
A) 0.07 kcal
B) 0.7 kcal
C) 6.1 kcal
D) 0.67 kcal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
How much heat is necessary to change 10 g of ice at -20°C into water at 10°C?
A) 0.02 kcal
B) 0.2 kcal
C) 1 kcal
D) 0.08 kcal
A) 0.02 kcal
B) 0.2 kcal
C) 1 kcal
D) 0.08 kcal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
The insulation in walls uses which of the following methods to prevent heat transfer?
A) Conduction
B) Convection
C) Radiation
D) Conduction and convection
E) All of these
A) Conduction
B) Convection
C) Radiation
D) Conduction and convection
E) All of these

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following has the highest thermal conductivity?
A) Air
B) A newspaper
C) A rug
D) An aluminum saucepan
A) Air
B) A newspaper
C) A rug
D) An aluminum saucepan

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
How much heat is necessary to change 20 g of water at 100°C into steam at 100°C?
A) 0.16 kcal
B) 10800 kcal
C) 10.8 kcal
D) 1.6 kcal
A) 0.16 kcal
B) 10800 kcal
C) 10.8 kcal
D) 1.6 kcal

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
The energy involved in a phase change is called the
A) specific heat.
B) thermal energy.
C) internal energy.
D) latent heat.
A) specific heat.
B) thermal energy.
C) internal energy.
D) latent heat.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
When heat is added to a closed system,it goes into
A) work only.
B) entropy only.
C) internal energy only.
D) either (A) or (C) or both.
A) work only.
B) entropy only.
C) internal energy only.
D) either (A) or (C) or both.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
The impossibility of reaching a temperature of absolute zero is incorporated in
A) the third law of thermodynamics.
B) the first law of thermodynamics.
C) the second law of thermodynamics.
D) an increase in entropy.
A) the third law of thermodynamics.
B) the first law of thermodynamics.
C) the second law of thermodynamics.
D) an increase in entropy.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
If the temperature of a quantity of ideal gas increases,then
A) the number of molecules must increase.
B) the pressure must increase.
C) the volume must increase.
D) the product of the pressure and volume must increase.
A) the number of molecules must increase.
B) the pressure must increase.
C) the volume must increase.
D) the product of the pressure and volume must increase.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
If 700 Joules of heat is added to a system,and the internal energy increases by 450 Joules,how much work is done by the system?
A) -1150 J
B) -250 J
C) 700 J
D) 250 J
E) 1150 J
A) -1150 J
B) -250 J
C) 700 J
D) 250 J
E) 1150 J

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is not directly involved in the second law of thermodynamics?
A) Spontaneous heat flow
B) Converting all heat input into work
C) Conservation of energy
D) Entropy
A) Spontaneous heat flow
B) Converting all heat input into work
C) Conservation of energy
D) Entropy

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
What happens to a sample of water when its temperature is reduced between 4°C and 100°C?
A) Its density increases.
B) It vaporizes.
C) Its density decreases.
D) Its density remains constant.
A) Its density increases.
B) It vaporizes.
C) Its density decreases.
D) Its density remains constant.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
What happens to a sample of water when its temperature is reduced between 0°C and 4°C?
A) Its density increases.
B) It vaporizes.
C) Its density decreases.
D) Its density remains constant.
A) Its density increases.
B) It vaporizes.
C) Its density decreases.
D) Its density remains constant.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
What happens to a sample of water when it is heated between 0°C and 4°C?
A) Its volume increases.
B) Its volume is reduced.
C) It vaporizes.
D) Nothing changes except its temperature.
A) Its volume increases.
B) Its volume is reduced.
C) It vaporizes.
D) Nothing changes except its temperature.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
For every natural process,the entropy of the universe
A) is destroyed.
B) decreases.
C) increases.
D) remains constant.
A) is destroyed.
B) decreases.
C) increases.
D) remains constant.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
If 700 Joules of heat is added to a system,while the system does 550 Joules,by how much does the internal energy increase?
A) -1250 J
B) -150 J
C) 700 J
D) 150 J
E) 1250 J
A) -1250 J
B) -150 J
C) 700 J
D) 150 J
E) 1250 J

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
When a material is found to have no definite volume and no definite shape,what is the phase of the material?
A) Gas
B) Liquid
C) Plasma
D) Solid
A) Gas
B) Liquid
C) Plasma
D) Solid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
A quantity that gives the direction of a thermodynamic process is
A) entropy.
B) efficiency.
C) specific heat.
D) energy.
A) entropy.
B) efficiency.
C) specific heat.
D) energy.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
At approximately what temperature does water have its greatest density?
A) 4°C
B) 100°C
C) 0°C
D) None of these; the density of water is constant at all temperatures.
A) 4°C
B) 100°C
C) 0°C
D) None of these; the density of water is constant at all temperatures.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
What happens to a sample of water when it is heated between 4°C and 100°C?
A) Its volume increases.
B) Its volume is reduced.
C) It vaporizes.
D) Nothing changes except its temperature.
A) Its volume increases.
B) Its volume is reduced.
C) It vaporizes.
D) Nothing changes except its temperature.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
When a material is found to have a definite volume and definite shape,and when it doesn't require a container to maintain its shape,what is the phase of the material?
A) Gas
B) Liquid
C) Plasma
D) Solid
A) Gas
B) Liquid
C) Plasma
D) Solid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
A constant volume and mass of helium gas at 77°C is heated so that the pressure of the gas doubles.What is the new temperature of the gas in Celsius degrees?
A) 154°C
B) 273°C
C) 427°C
D) 700°C
A) 154°C
B) 273°C
C) 427°C
D) 700°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
A ______________ is an ultra-hot collection of electrically charged particles.
A) gas
B) liquid
C) plasma
D) solid
A) gas
B) liquid
C) plasma
D) solid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
Which laboratory observation would most support the kinetic theory's assumption that molecules in a gas are widely separated?
A) A gas exerts the same pressure on each part of a container's wall.
B) A gas is highly compressible.
C) A gas with a lower formula mass diffuses more rapidly than one with a higher formula mass.
D) A gas expands when the pressure on it is released.
A) A gas exerts the same pressure on each part of a container's wall.
B) A gas is highly compressible.
C) A gas with a lower formula mass diffuses more rapidly than one with a higher formula mass.
D) A gas expands when the pressure on it is released.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
A heat engine
A) converts heat to work.
B) converts heat to internal energy.
C) transfers heat from a low-temperature reservoir.
D) converts work to heat.
A) converts heat to work.
B) converts heat to internal energy.
C) transfers heat from a low-temperature reservoir.
D) converts work to heat.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
When a material is found to have a definite volume but no definite shape,and when it assumes the shape of the container in which it is placed,what is the phase of the material?
A) Gas
B) Liquid
C) Plasma
D) Solid
A) Gas
B) Liquid
C) Plasma
D) Solid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
On bare feet,a tile floor feels colder than a rug because the tile has a greater ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
The Fahrenheit scale has a ______________ degree size than the Celsius scale.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
The SI unit of heat is the ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
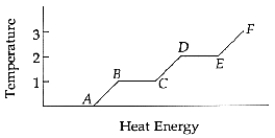
The substance is entirely a liquid between points ______________ and ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
Solids,liquids,and gases are called ______________ of matter.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
The ice point and the steam point of water are commonly called the ______________ point and the ______________ point,respectively.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
A common property used to measure temperature is the ______________ of materials.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
The amount of heat necessary to change a liquid to a solid at constant temperature is the ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
In a heat pump,
A) heat is used to do work.
B) heat flows spontaneously.
C) the same process is used as in a heat engine.
D) work is used to transfer heat.
A) heat is used to do work.
B) heat flows spontaneously.
C) the same process is used as in a heat engine.
D) work is used to transfer heat.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
Water has a specific heat value of ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
Below 4°C,water ______________ with decreasing temperature.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
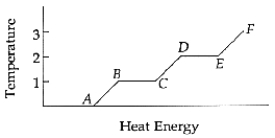
The latent heat of fusion is the amount of energy per kilogram necessary to go from point ______________ to point ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
The ______________ temperature scale has no negative readings.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
The energy needed to change the phase of a substance is called ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
Gas pressure is caused by molecular ______________ with the walls of the container.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
A ______________ has a definite volume and assumes the shape of its container.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
______________ is the process whereby energetic molecules escape from a liquid.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
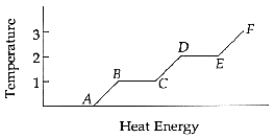
The substance is entirely a gas between points ______________ and ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
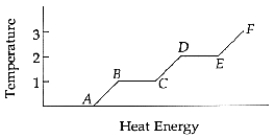
Temperatures 1 and 2 are the ______________ and the ______________,respectively.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
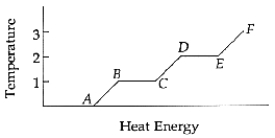
The substance is entirely a solid between points ______________ and ______________.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck


