Deck 5: Ground Rules of Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/69
Play
Full screen (f)
Deck 5: Ground Rules of Metabolism
1
The active site of an enzyme ____.
A) is where the coenzyme is located
B) is a specific bulge or protuberance on an enzyme
C) is a groove or crevice in the structure of the enzyme complementary to the substrate
D) will react with only one substrate no matter how many molecules may resemble the shape of the substrate
E) rigidly resists any alteration of its shape
A) is where the coenzyme is located
B) is a specific bulge or protuberance on an enzyme
C) is a groove or crevice in the structure of the enzyme complementary to the substrate
D) will react with only one substrate no matter how many molecules may resemble the shape of the substrate
E) rigidly resists any alteration of its shape
C
2
Endergonic reactions ____.
A) result in products with less energy than the reactants
B) require a net input of energy
C) occur in the breakdown of glucose
D) are used by cells to provide energy for biological reactions
E) break down large molecules into smaller molecules
A) result in products with less energy than the reactants
B) require a net input of energy
C) occur in the breakdown of glucose
D) are used by cells to provide energy for biological reactions
E) break down large molecules into smaller molecules
B
3
What is the key activity of panel II in the accompanying figure?
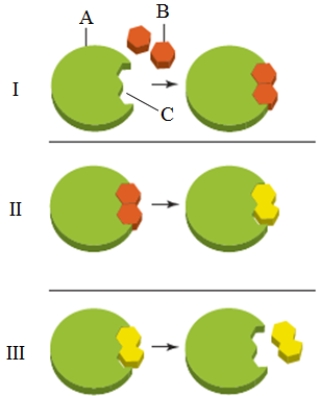
A) The enzyme is consumed as the products form.
B) The substrate leaves the active site.
C) The conversion of substrates to products occurs.
D) The product leaves the enzyme.
E) The product decomposes to the reactants.

A) The enzyme is consumed as the products form.
B) The substrate leaves the active site.
C) The conversion of substrates to products occurs.
D) The product leaves the enzyme.
E) The product decomposes to the reactants.
C
4
The activation energy of a reaction refers to the minimum amount of energy ____.
A) released by the reaction
B) in the reactants
C) in the products
D) necessary to cause a reaction to proceed on its own
E) variation between the energy of the reactants and the energy of the products
A) released by the reaction
B) in the reactants
C) in the products
D) necessary to cause a reaction to proceed on its own
E) variation between the energy of the reactants and the energy of the products

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
5
What is the item labeled "C" in the accompanying figure?
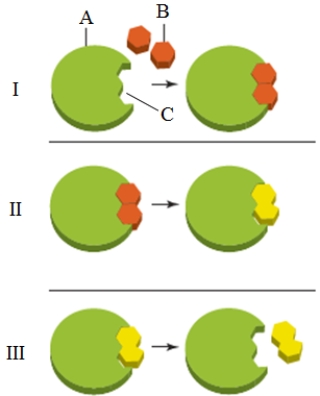
A) The reactant
B) The substrate
C) The active site
D) The transition state
E) The product

A) The reactant
B) The substrate
C) The active site
D) The transition state
E) The product

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
6
What is the item labeled "B" in the accompanying figure?
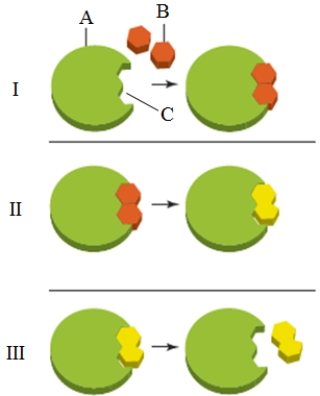
A) The enzyme
B) The substrate
C) The active site
D) The transition state
E) The product

A) The enzyme
B) The substrate
C) The active site
D) The transition state
E) The product

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
7
Enzymes ____.
A) control the speed of a reaction
B) change shapes to facilitate certain reactions.
C) may place physical stress on the bonds of the substrate.
D) always work on their own.
E) lower activation energy.
A) control the speed of a reaction
B) change shapes to facilitate certain reactions.
C) may place physical stress on the bonds of the substrate.
D) always work on their own.
E) lower activation energy.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
8
An enzyme lowers activation energy by ____.
A) keeping substrates apart
B) orienting substrates randomly
C) breaking the laws of thermodynamics
D) shutting out water molecules
E) physically breaking chemical bonds
A) keeping substrates apart
B) orienting substrates randomly
C) breaking the laws of thermodynamics
D) shutting out water molecules
E) physically breaking chemical bonds

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
9
According to the first law of thermodynamics, ____.
A) the energy of a system always increases
B) the amount of energy in the universe is changeable
C) chemical reactions do not create or destroy energy
D) energy is always stable
E) energy always decreases
A) the energy of a system always increases
B) the amount of energy in the universe is changeable
C) chemical reactions do not create or destroy energy
D) energy is always stable
E) energy always decreases

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
10
An enzyme is thought to optimize the fit between substrates by restraining and stretching or squeezing them into certain shapes and moving them to the transition state, as described by the ____ model of enzyme activity.
A) lock and key
B) induced-fit
C) template
D) activation
E) conformational
A) lock and key
B) induced-fit
C) template
D) activation
E) conformational

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
11
What are the effects of long term excessive alcohol consumption on the liver?
A) fat accumulation and abnormal discoloration of the liver
B) scarring, hardening, and increased fat metabolism
C) increased ability to degrade fats and toxins
D) decreased production of ethanol and cholesterol
E) scarring, hardening, and fat accumulation
A) fat accumulation and abnormal discoloration of the liver
B) scarring, hardening, and increased fat metabolism
C) increased ability to degrade fats and toxins
D) decreased production of ethanol and cholesterol
E) scarring, hardening, and fat accumulation

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
12
What is a key characteristic of enzyme behavior?
A) Enzyme shape is stable during catalysis.
B) The active site of an enzyme orients its substrate molecules, thereby promoting interaction of their reactive parts.
C) All enzymes have an active site where coenzymes are temporarily bound.
D) Each enzyme can catalyze multiple reactions.
E) Enzyme activity is not affected by pH and temperature.
A) Enzyme shape is stable during catalysis.
B) The active site of an enzyme orients its substrate molecules, thereby promoting interaction of their reactive parts.
C) All enzymes have an active site where coenzymes are temporarily bound.
D) Each enzyme can catalyze multiple reactions.
E) Enzyme activity is not affected by pH and temperature.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
13
Energy is commonly defined as ____.
A) a form of heat
B) thermodynamics
C) the capacity to change
D) the capacity to do work
E) the ability to do work
A) a form of heat
B) thermodynamics
C) the capacity to change
D) the capacity to do work
E) the ability to do work

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
14
CO₂ and H₂O will not form glucose on their own because ____.
A) CO₂ does not contain sufficient energy
B) H₂O does not contain sufficient energy
C) neither CO₂ nor H₂O contain sufficient energy
D) the concentration of CO₂ is too low in the atmosphere
E) the bonds of CO₂ and H₂O are too stable to be broken without an input of energy
A) CO₂ does not contain sufficient energy
B) H₂O does not contain sufficient energy
C) neither CO₂ nor H₂O contain sufficient energy
D) the concentration of CO₂ is too low in the atmosphere
E) the bonds of CO₂ and H₂O are too stable to be broken without an input of energy

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
15
The second law of thermodynamics states that ____.
A) energy can be transformed into matter, and because of this, something is obtained for nothing
B) energy can be destroyed during nuclear reactions such as those that occur inside the sun
C) if energy is gained by one region of the universe, another place in the universe also must gain energy in order to maintain the balance of nature
D) energy tends to flow from concentrated to less concentrated forms
E) energy tends to be stable over time
A) energy can be transformed into matter, and because of this, something is obtained for nothing
B) energy can be destroyed during nuclear reactions such as those that occur inside the sun
C) if energy is gained by one region of the universe, another place in the universe also must gain energy in order to maintain the balance of nature
D) energy tends to flow from concentrated to less concentrated forms
E) energy tends to be stable over time

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
16
In order to work best, enzymes ____.
A) always need coenzymes
B) randomly grab surrounding molecules
C) need low pH
D) need high temperatures
E) need set environmental conditions, specific for each enzyme
A) always need coenzymes
B) randomly grab surrounding molecules
C) need low pH
D) need high temperatures
E) need set environmental conditions, specific for each enzyme

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
17
The enzyme responsible for breaking down alcohol is ____.
A) alcohol methylase
B) alcohol polyphosphorylase
C) hydroxyl alcoholgenase
D) transmethylogenase
E) alcohol dehydrogenase
A) alcohol methylase
B) alcohol polyphosphorylase
C) hydroxyl alcoholgenase
D) transmethylogenase
E) alcohol dehydrogenase

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
18
What type of energy is the energy in chemical bonds?
A) solar energy
B) activation energy
C) kinetic energy
D) potential energy
E) thermal energy
A) solar energy
B) activation energy
C) kinetic energy
D) potential energy
E) thermal energy

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
19
What statement is an application of the first law of thermodynamics?
A) The level of entropy increases as time passes.
B) Living organisms represent an exception to the laws of energy.
C) The quantity of energy does not increase or decrease in the universe.
D) Fungi and plants do not make their own energy but derive it from somewhere else.
E) The amount of energy found in the compounds on one side of an equation is equal to that on the other side.
A) The level of entropy increases as time passes.
B) Living organisms represent an exception to the laws of energy.
C) The quantity of energy does not increase or decrease in the universe.
D) Fungi and plants do not make their own energy but derive it from somewhere else.
E) The amount of energy found in the compounds on one side of an equation is equal to that on the other side.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
20
What is a key characteristic of exergonic reactions?
A) They consume energy.
B) They require glucose fusion.
C) Their products have more energy than the reactants.
D) They convert some energy to less biologically useful forms.
E) They involve the formation of bonds.
A) They consume energy.
B) They require glucose fusion.
C) Their products have more energy than the reactants.
D) They convert some energy to less biologically useful forms.
E) They involve the formation of bonds.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
21
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
active site
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
active site

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
22
The rate of diffusion through a selectively permeable membrane will be lowest when ____.
I)concentration gradients are steep
II)temperatures are low
III)solutes are small molecules
A) I only
B) II only
C) I and III
D) II and III
E) I, II, and III
I)concentration gradients are steep
II)temperatures are low
III)solutes are small molecules
A) I only
B) II only
C) I and III
D) II and III
E) I, II, and III

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
23
In some cases, inhibitors or activators of enzyme-catalyzed reactions act by ____.
A) binding to the substrates
B) affecting the supply of ATP
C) reversibly binding to an enzyme's allosteric site
D) reducing or increasing the concentration of enzymes
E) binding to the products
A) binding to the substrates
B) affecting the supply of ATP
C) reversibly binding to an enzyme's allosteric site
D) reducing or increasing the concentration of enzymes
E) binding to the products

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
24
During receptor-mediated endocytosis, ____.
A) plasma membrane receptors bind molecules to be expelled
B) a small channel forms beneath the receptors
C) a pit opens inside a cell
D) a vesicle forms
E) a vesicle is destroyed
A) plasma membrane receptors bind molecules to be expelled
B) a small channel forms beneath the receptors
C) a pit opens inside a cell
D) a vesicle forms
E) a vesicle is destroyed

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
25
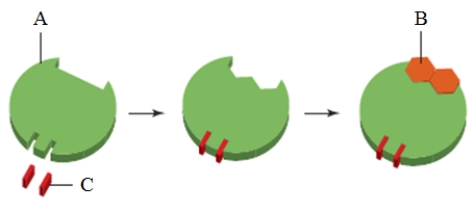
The figure above represents ____.
A) an exergonic reaction
B) a metabolic pathway
C) allosteric inhibition
D) allosteric regulation
E) an endergonic reaction

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
26
The action of a white blood cell engulfing a bacterium is known as ____.
A) pinocytosis only
B) phagocytosis only
C) exocytosis and phagocytosis
D) endocytosis and pinocytosis
E) phagocytosis and endocytosis
A) pinocytosis only
B) phagocytosis only
C) exocytosis and phagocytosis
D) endocytosis and pinocytosis
E) phagocytosis and endocytosis

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
27
What method of movement requires the expenditure of ATP molecules?
A) simple diffusion
B) facilitated diffusion
C) osmosis
D) active transport
E) bulk flow
A) simple diffusion
B) facilitated diffusion
C) osmosis
D) active transport
E) bulk flow

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
28
Allosteric enzymes ____.
A) have regions that bind with inhibitor or activator molecules
B) are associated with important energy-carrying nucleotides
C) are not affected by temperature or pH
D) have two active sites
E) have a broader range of working conditions
A) have regions that bind with inhibitor or activator molecules
B) are associated with important energy-carrying nucleotides
C) are not affected by temperature or pH
D) have two active sites
E) have a broader range of working conditions

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
29
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
enzyme
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
enzyme

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
30
What term best describes the role of certain metal ions and coenzymes in metabolic processes?
A) reactants
B) intermediates
C) cofactors
D) products
E) catalysts
A) reactants
B) intermediates
C) cofactors
D) products
E) catalysts

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
31
In oxidation-reduction reactions, ____.
A) both molecules give up electrons
B) both molecules gain electrons
C) cells harvest energy
D) the molecule that accepts electrons is reduced
E) hydrogen ions are usually absorbed
A) both molecules give up electrons
B) both molecules gain electrons
C) cells harvest energy
D) the molecule that accepts electrons is reduced
E) hydrogen ions are usually absorbed

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
32
A "high-energy bond" in ATP ____.
A) absorbs a large amount of free energy when the phosphate group is attached during hydrolysis
B) is formed when ATP is hydrolyzed to ADP and one phosphate group
C) is similar to the bonds in glucose molecules
D) forms as part of an endergonic reaction
E) cannot be readily used in reactions
A) absorbs a large amount of free energy when the phosphate group is attached during hydrolysis
B) is formed when ATP is hydrolyzed to ADP and one phosphate group
C) is similar to the bonds in glucose molecules
D) forms as part of an endergonic reaction
E) cannot be readily used in reactions

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
33
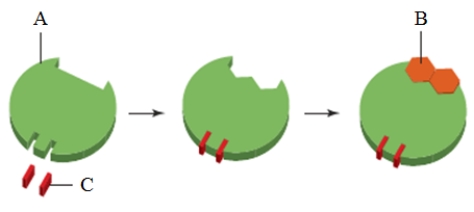
In the image above, the substance indicated as "B" is a(n) ____.
A) inhibitor
B) regulatory molecule
C) substrate
D) coenzyme
E) cofactor

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
34
What affects the rate of diffusion through a selectively permeable membrane?
I)concentration gradient
II)temperature
III)molecular size
A) I only
B) II only
C) I and II
D) II and III
E) I, II, and III
I)concentration gradient
II)temperature
III)molecular size
A) I only
B) II only
C) I and II
D) II and III
E) I, II, and III

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement below characterizes metabolic pathways?
A) They are a random sequences of reactions.
B) They are always biosynthetic.
C) They are always degradative.
D) They are enzyme-mediated sequences of reactions.
E) They are always linear reactions.
A) They are a random sequences of reactions.
B) They are always biosynthetic.
C) They are always degradative.
D) They are enzyme-mediated sequences of reactions.
E) They are always linear reactions.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
36
The mosaic of the fluid mosaic model refers to ____.
A) specific proteins in membranes
B) the liquid inside a cell
C) the phospholipids in membranes
D) the passage of materials into cells
E) the diverse molecules making up membranes
A) specific proteins in membranes
B) the liquid inside a cell
C) the phospholipids in membranes
D) the passage of materials into cells
E) the diverse molecules making up membranes

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
37
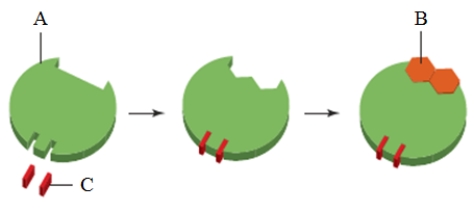 In the image above, the substance indicated as "C" is a(n) _____.
In the image above, the substance indicated as "C" is a(n) _____.A) inhibitor
B) regulatory molecule
C) substrate
D) coenzyme
E) cofactor

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
38
Allosteric inhibition generally results from ____.
A) excess substrates
B) binding of regulatory molecules at sites other than the active sites
C) a change in the temperature of the system
D) a lack of coenzymes
E) changes in pH
A) excess substrates
B) binding of regulatory molecules at sites other than the active sites
C) a change in the temperature of the system
D) a lack of coenzymes
E) changes in pH

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
39
During the process of phosphorylation, ____.
A) a molecule gains a phosphate group
B) a molecule synthesizes a phosphate group
C) molecules become more stable
D) molecules are passed to enzymes
E) ADP is produced from ATP
A) a molecule gains a phosphate group
B) a molecule synthesizes a phosphate group
C) molecules become more stable
D) molecules are passed to enzymes
E) ADP is produced from ATP

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a form of active transport?
A) osmosis
B) facilitated diffusion
C) simple diffusion
D) exocytosis
E) glucose transport
A) osmosis
B) facilitated diffusion
C) simple diffusion
D) exocytosis
E) glucose transport

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
41
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of molecules across a membrane would be equal
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of molecules across a membrane would be equal

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
42
Classification. The items below are processes that occur during different cellular reaction or metabolic pathways. Answer the following questions by selecting the correct term
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process involves a series of enzymes and requires membranes.
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process involves a series of enzymes and requires membranes.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
43
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of molecules would be out of this fluid
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of molecules would be out of this fluid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
44
Classification. The items below are processes that occur during different cellular reaction or metabolic pathways. Answer the following questions by selecting the correct term
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process leads to the formation of ATP from ADP plus inorganic phosphate.
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process leads to the formation of ATP from ADP plus inorganic phosphate.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
45
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
fluid with a high solute concentration relative to another fluid
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
fluid with a high solute concentration relative to another fluid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
46
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
entropy
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
entropy

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
47
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
allosteric enzyme
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
allosteric enzyme

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
48
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
adenosine triphosphate
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
adenosine triphosphate

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
49
Classification. The items below are processes that occur during different cellular reaction or metabolic pathways. Answer the following questions by selecting the correct term
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process occurs when an electron is received by an electron acceptor molecule, such as NADP+.
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process occurs when an electron is received by an electron acceptor molecule, such as NADP+.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
50
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
feedback inhibition
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
feedback inhibition

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
51
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
activation energy
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
activation energy

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
52
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of molecules would be into this fluid
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of molecules would be into this fluid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
53
Classification. The items below are processes that occur during different cellular reaction or metabolic pathways. Answer the following questions by selecting the correct term
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process occurs when an electron transport molecule gives up an electron.
a.oxidation
b.reduction
c.phosphorylation
d.electron transport chain
This process occurs when an electron transport molecule gives up an electron.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
54
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
catalase
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
catalase

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
55
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
pressure that prevents the movement of water
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
pressure that prevents the movement of water

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
56
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of water molecules across a membrane
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
movement of water molecules across a membrane

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
57
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
phosphorylation
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
phosphorylation

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
58
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
pressure from a fluid against a boundary
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
pressure from a fluid against a boundary

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
59
Matching
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
NADP+
Choose the one most appropriate answer for each.
a.amount of energy to start a reaction
b.coenzyme that carries electrons
c.attachment of a phosphate group by a high-energy bond
d.an excess of end-product molecules alters the shape of the first enzyme in the pathway and shuts off that metabolic pathway
e.part of an enzyme that binds to the substrate
f.by binding a regulatory molecule, it changes the activity of a metabolic pathway
g.affects rates of chemical reactions by reducing activation energy
h.important energy currency in metabolism
i.enzyme that participates in the neutralization of hydrogen peroxide
j.a measure of the degree of energy change after a concentrated form of energy has been dispersed
NADP+

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
60
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
fluid with a low solute concentration relative to another fluid
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
fluid with a low solute concentration relative to another fluid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
61
A chemical interaction in which the products contain less free energy than the reactants is called a(n) ____________________ reaction.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
62
____________________ enzymes are controlled by having regulatory molecules bind outside the active site and result in a change in their overall structure.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
63
Describe how the laws of thermodynamics would impact the Earth were the sun's light suddenly blocked.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
64
The enzyme that works in conjunction with ATP to produce bioluminescence is called ____________________.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
65
Discuss the role of turgor in the crisp lettuce purchased at the grocery store looking much limper by the time it gets home.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
66
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
spontaneous movement of molecules
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
spontaneous movement of molecules

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
67
Compare and contrast facilitated and active transport.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
68
Classification. The terms below relate to the movement of materials and membranes. Answer the following questions by selecting the correct term.
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
fluid with the same solute concentration relative to another fluid
a.diffusion
b.hypertonic
c.hypotonic
d.isotonic
e.osmosis
f.osmotic pressure
g.turgor
fluid with the same solute concentration relative to another fluid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
69
The loss of an electron by a molecule is called ____________________.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck


