Deck 13: Mechanisms of Enzyme Action
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/27
Play
Full screen (f)
Deck 13: Mechanisms of Enzyme Action
1
Which of the following values is the estimated approximate lifetime of the transition state?
A)microseconds (10-6 s)
B)nanoseconds (10-9 s)
C)(10-14 to 10-13 s)
D)(10-20 s)
A)microseconds (10-6 s)
B)nanoseconds (10-9 s)
C)(10-14 to 10-13 s)
D)(10-20 s)
C
2
Which of the following structures has the greatest complementarity to enzyme active sites?
A)substrate
B)transition state
C)product
D)stable intermediate
A)substrate
B)transition state
C)product
D)stable intermediate
B
3
The catalytic triad common to many serine proteases involves shuttling of protons between the amino acids in the catalytic triad.Which of the following amino acid sequences is in the correct order for this process?
A)Ser-His-Asp
B)His-Ser-Asp
C)Ser-His-His
D)Asp-His-Ser
A)Ser-His-Asp
B)His-Ser-Asp
C)Ser-His-His
D)Asp-His-Ser
A
4
Which of the following is NOT a catalytic mechanism or factor that contributes to the performance of enzymes?
A)entropy gain in ES formation
B)covalent catalysis
C)general acid or base catalysis
D)proximity and orientation
A)entropy gain in ES formation
B)covalent catalysis
C)general acid or base catalysis
D)proximity and orientation

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following regarding the hydrolysis of p-nitrophenyl acetate by chymotrypsin is correct?
A)The active site contains a serine residue,an unprotonated histidine,and an unprotonated aspartate residue at pH 7.0 prior to binding the substrate.
B)The first product is acetic acid.
C)An actyl-enzyme tetrahedral intermediate forms during the mechanism.
D)Attack of a water molecule on the acyl-enzyme intermediate yields the first product.
A)The active site contains a serine residue,an unprotonated histidine,and an unprotonated aspartate residue at pH 7.0 prior to binding the substrate.
B)The first product is acetic acid.
C)An actyl-enzyme tetrahedral intermediate forms during the mechanism.
D)Attack of a water molecule on the acyl-enzyme intermediate yields the first product.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following inhibitors is effective as a transition-state analog?
A)competitive
B)non-competitive
C)allosteric
D)irreversible
A)competitive
B)non-competitive
C)allosteric
D)irreversible

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is NOT a characteristic of metal ion catalysis?
A)increased acidity of a nucleophile with an ionizable proton
B)metal ion requirement to maintain the stable,native state of the enzyme
C)strongly metal binding,perhaps not only during the catalytic cycle
D)electrophilic catalysis,stabilizing the increased electron density or negative charge that can develop during a reaction
A)increased acidity of a nucleophile with an ionizable proton
B)metal ion requirement to maintain the stable,native state of the enzyme
C)strongly metal binding,perhaps not only during the catalytic cycle
D)electrophilic catalysis,stabilizing the increased electron density or negative charge that can develop during a reaction

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following types of catalytic mechanisms classifies the reaction below?
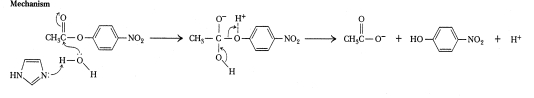
A)covalent nucleophilic
B)covalent electrophilic
C)specific base catalysis
D)general base catalysis

A)covalent nucleophilic
B)covalent electrophilic
C)specific base catalysis
D)general base catalysis

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following types of kinetic mechanisms is used by enzymes carrying out covalent catalysis?
A)Ping-Pong
B)sequential bisubstrate
C)random bisubstrate
D)simple unimolecular
A)Ping-Pong
B)sequential bisubstrate
C)random bisubstrate
D)simple unimolecular

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following determines the enzymatic reaction rate?
A)the difference in energy between ES and S
B)the difference in energy between ES and P
C)the difference in energy between ES and EX‡
D)the difference in energy between ES and ESI
A)the difference in energy between ES and S
B)the difference in energy between ES and P
C)the difference in energy between ES and EX‡
D)the difference in energy between ES and ESI

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following attributes does NOT explain why enzymes are flexible proteins and NOT rigid molecules?
A)Active sites are able to conform to the shape of the substrate.
B)Strain is placed upon the substrate while it binds to the enzyme.
C)Catalytic residues are oriented in such a way that they play a minor role in bond breakage and bond formation.
D)Near-attack conformations are achieved during formation of the transition state during an enzyme-catalyzed reaction.
A)Active sites are able to conform to the shape of the substrate.
B)Strain is placed upon the substrate while it binds to the enzyme.
C)Catalytic residues are oriented in such a way that they play a minor role in bond breakage and bond formation.
D)Near-attack conformations are achieved during formation of the transition state during an enzyme-catalyzed reaction.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following will increase the energy level of ES and subsequently the enzyme-catalyzed reaction rate?
A)stabilization of ES by solvation
B)destabilization of ES by strain
C)stabilization of ES by hydrophobic effects
D)destabilization of ES by electrostatic effects
A)stabilization of ES by solvation
B)destabilization of ES by strain
C)stabilization of ES by hydrophobic effects
D)destabilization of ES by electrostatic effects

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following regarding for low-barrier hydrogen bonds is correct?
A)The barrier that the hydrogen atom must surmount to exchange oxygens becomes higher.
B)The interactions are not like covalent bonds.
C)The bond order approaches 2 for both O-H interactions.
D)The hydrogen is centred between the 2 heteroatoms.
A)The barrier that the hydrogen atom must surmount to exchange oxygens becomes higher.
B)The interactions are not like covalent bonds.
C)The bond order approaches 2 for both O-H interactions.
D)The hydrogen is centred between the 2 heteroatoms.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following amino acid side chains is often involved in general acid-base catalysis because its pKₐ is near 7?
A)cysteine
B)aspartate
C)histidine
D)lysine
A)cysteine
B)aspartate
C)histidine
D)lysine

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following types of serine protease inhibitors are organic fluorophosphates?
A)competitive
B)uncompetitive
C)non-competitive
D)irreversible
A)competitive
B)uncompetitive
C)non-competitive
D)irreversible

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following regarding the enzyme-transition state complex is correct?
A)The enzyme is designed to bind the transition-state structure less tightly than the substrate or product.
B)It is designated as EX‡.
C)The enzyme stabilizes the transition-state complex less than it stabilizes the substrate complex.
D)The energy barrier between ES and EX‡ is more than the energy barrier between S and X‡.
A)The enzyme is designed to bind the transition-state structure less tightly than the substrate or product.
B)It is designated as EX‡.
C)The enzyme stabilizes the transition-state complex less than it stabilizes the substrate complex.
D)The energy barrier between ES and EX‡ is more than the energy barrier between S and X‡.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a feature of transition-state analogs?
A)They are approximations of the transition state that bind less tightly than the substrate.
B)They are compounds that compete for the active site and are always very similar to the substrate.
C)They are stable molecules that are chemically and structurally similar to the transition state.
D)They are stable molecules that can be expected to resemble the true transition state very closely.
A)They are approximations of the transition state that bind less tightly than the substrate.
B)They are compounds that compete for the active site and are always very similar to the substrate.
C)They are stable molecules that are chemically and structurally similar to the transition state.
D)They are stable molecules that can be expected to resemble the true transition state very closely.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following elements is involved in the mechanism of chymotrypsin?
A)deprotonation of an active site Asp residue by His to start the reaction
B)formation of an acyl-enzyme intermediate that must be hydrolyzed to complete the reaction
C)stabilization of the positively charged His by a Gln residue
D)direct deprotonation of water by His to generate a hydroxide ion for initiation of the reaction
A)deprotonation of an active site Asp residue by His to start the reaction
B)formation of an acyl-enzyme intermediate that must be hydrolyzed to complete the reaction
C)stabilization of the positively charged His by a Gln residue
D)direct deprotonation of water by His to generate a hydroxide ion for initiation of the reaction

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following amino acid side chains are NOT found in nucleophilic centres used for covalent catalysis?
A)methyl
B)amines
C)carboxylate
D)aryl and alkyl hydroxyls
A)methyl
B)amines
C)carboxylate
D)aryl and alkyl hydroxyls

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements regarding enzymes and transition states is correct?
A)Stabilization of the transition state must be less than stabilization of ES for catalysis to occur.
B)Binding of substrate to an enzyme often causes strain,thus promoting transition-state formation.
C)The transition-state conformation of an enzyme-catalyzed reaction is identical to the conformation seen in the uncatalyzed transition state.
D)Formation of the transition state always assures that the reaction will proceed to product.
A)Stabilization of the transition state must be less than stabilization of ES for catalysis to occur.
B)Binding of substrate to an enzyme often causes strain,thus promoting transition-state formation.
C)The transition-state conformation of an enzyme-catalyzed reaction is identical to the conformation seen in the uncatalyzed transition state.
D)Formation of the transition state always assures that the reaction will proceed to product.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
21
Aspartate proteases display a variety of substrate specificities.Which of the following peptide bonds,however,do they most actively cleave?
A)those on the carboxyl side of the basic amino acids
B)those on the amino side of aromatic amino acids
C)those between 2 small,neutral residues
D)those between 2 hydrophobic amino acid residues
A)those on the carboxyl side of the basic amino acids
B)those on the amino side of aromatic amino acids
C)those between 2 small,neutral residues
D)those between 2 hydrophobic amino acid residues

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the proposed mechanism of aspartate protease catalysis?
A)covalent nucleophilic
B)covalent electrophilic facilitated by a low-barrier hydrogen bond
C)specific base catalysis
D)general base-general acid facilitated by a low-barrier hydrogen bond
A)covalent nucleophilic
B)covalent electrophilic facilitated by a low-barrier hydrogen bond
C)specific base catalysis
D)general base-general acid facilitated by a low-barrier hydrogen bond

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is NOT a characteristic of an effective HIV-1 protease inhibitor?
A)effective delivery in sufficient quantities to the desired site(s)of action in the organism
B)broad spectrum enough to be effective against mutant viral forms
C)a backbone -OH group that forms a weak association with the 2 active-site carboxyl groups of the protease
D)results in inhibiting the production of new virus particles in cells of infected patients
A)effective delivery in sufficient quantities to the desired site(s)of action in the organism
B)broad spectrum enough to be effective against mutant viral forms
C)a backbone -OH group that forms a weak association with the 2 active-site carboxyl groups of the protease
D)results in inhibiting the production of new virus particles in cells of infected patients

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following pairs below forms a low-barrier hydrogen bond?
A)Asp102 and Ser195
B)Asp102 and His57
C)His57 and Ser195
D)Ser195 and carbonyl oxygen in the peptide bond
A)Asp102 and Ser195
B)Asp102 and His57
C)His57 and Ser195
D)Ser195 and carbonyl oxygen in the peptide bond

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
25
Where is the initial bond formation located in the covalent intermediate in the chymotrypsin-catalyzed reaction?
A)between serine and the carbonyl carbon in the peptide backbone
B)between nitrogen and the carbonyl carbon in the peptide backbone
C)between histidine and the carbonyl carbon in the peptide backbone
D)between aspartate and the carbonyl carbon in the peptide backbone
A)between serine and the carbonyl carbon in the peptide backbone
B)between nitrogen and the carbonyl carbon in the peptide backbone
C)between histidine and the carbonyl carbon in the peptide backbone
D)between aspartate and the carbonyl carbon in the peptide backbone

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
26
How does HIV-1 protease differ from most mammalian aspartic acid proteases?
A)It has 2 subunits,each with a 2-aspartate active site.
B)It has 2 subunits,each contributing an aspartate to the active site.
C)It has 2 active sites on 1 protein.
D)It has 2 subunits,1 with an active site and the other with a regulatory activity.
A)It has 2 subunits,each with a 2-aspartate active site.
B)It has 2 subunits,each contributing an aspartate to the active site.
C)It has 2 active sites on 1 protein.
D)It has 2 subunits,1 with an active site and the other with a regulatory activity.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is NOT relevant to the reaction catalyzed by chorismate mutase?
A)The reaction involves a concerted intramolecular rearrangement of chorismate to prephenate during the synthesis of phenylalanine.
B)The enzyme-catalyzed and uncatalyzed reactions follow almost identical routes.
C)The enzymatic reaction is thought to involve transition-state stabilization by 12 electrostatic and hydrogen bond interactions.
D)The geometry of the enzyme active site is such that the difference in energy between ES and the near attack conformation is more than 100 kJ/mol.
A)The reaction involves a concerted intramolecular rearrangement of chorismate to prephenate during the synthesis of phenylalanine.
B)The enzyme-catalyzed and uncatalyzed reactions follow almost identical routes.
C)The enzymatic reaction is thought to involve transition-state stabilization by 12 electrostatic and hydrogen bond interactions.
D)The geometry of the enzyme active site is such that the difference in energy between ES and the near attack conformation is more than 100 kJ/mol.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck


