Deck 2: Fundamental Building Blocks: Chemistry, water, and Ph
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 2: Fundamental Building Blocks: Chemistry, water, and Ph
1
A polar covalent bond results when:
A)two atoms share electrons equally.
B)two atoms of the same element are sharing electrons.
C)one of the atoms sharing electrons is more electronegative than the other atom.
D)two atoms sharing electrons are equally electronegative.
A)two atoms share electrons equally.
B)two atoms of the same element are sharing electrons.
C)one of the atoms sharing electrons is more electronegative than the other atom.
D)two atoms sharing electrons are equally electronegative.
D
2
An atom whose atomic number is 10 has how many electrons in its outermost energy level?
A)eight
B)ten
C)two
D)three
E)five
A)eight
B)ten
C)two
D)three
E)five
A
3
An element with 22 protons,22 neutrons,and 22 electrons would have an atomic number of:
A)44.
B)22.
C)66.
D)11.
A)44.
B)22.
C)66.
D)11.
B
4
Nonpolar molecules develop when:
A)shared electrons are not shared equally.
B)both atoms have similar electronegativity.
C)one atom is much more electronegative than the other.
D)electrons are completely transferred from one atom to another.
A)shared electrons are not shared equally.
B)both atoms have similar electronegativity.
C)one atom is much more electronegative than the other.
D)electrons are completely transferred from one atom to another.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
As the difference in the electronegativity between atoms forming a chemical bond increases,the:
A)less polar the molecule.
B)more polar the molecule.
C)more stable the molecule.
D)more symmetrical the molecule.
A)less polar the molecule.
B)more polar the molecule.
C)more stable the molecule.
D)more symmetrical the molecule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
All the atoms of the same element will have the same:
A)number of protons.
B)number of neutrons.
C)number of protons and neutrons.
D)mass.
A)number of protons.
B)number of neutrons.
C)number of protons and neutrons.
D)mass.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
If a neutral atom has an atomic number of 10,then we know that it has:
A)10 neutrons.
B)10 protons.
C)10 electrons.
D)10 protons and 10 electrons.
E)10 protons, 10 electrons, and 10 neutrons.
A)10 neutrons.
B)10 protons.
C)10 electrons.
D)10 protons and 10 electrons.
E)10 protons, 10 electrons, and 10 neutrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Chlorine has an atomic number of 17,and argon has an atomic number of 18.From this information alone,you can predict that:
A)argon has more neutrons than chlorine.
B)argon is more chemically reactive than chlorine.
C)argon will more readily ionize than chlorine.
D)chlorine is more chemically reactive than argon.
E)chlorine has more neutrons than argon.
A)argon has more neutrons than chlorine.
B)argon is more chemically reactive than chlorine.
C)argon will more readily ionize than chlorine.
D)chlorine is more chemically reactive than argon.
E)chlorine has more neutrons than argon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
What is it about carbon-12,carbon-13,and carbon-14 that makes them all carbon?
A)They all have the number of protons plus neutrons that is characteristic of carbon.
B)They all have the number of protons that is characteristic of carbon.
C)They all have the number of neutrons that is characteristic of carbon.
D)They all are radioactive.
E)They all are elements.
A)They all have the number of protons plus neutrons that is characteristic of carbon.
B)They all have the number of protons that is characteristic of carbon.
C)They all have the number of neutrons that is characteristic of carbon.
D)They all are radioactive.
E)They all are elements.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
A measure of the quantity of matter in an object is known as:
A)atoms.
B)density.
C)mass.
D)energy.
A)atoms.
B)density.
C)mass.
D)energy.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
For an atom to be considered an ion:
A)protons can outnumber neutrons.
B)protons can outnumber electrons.
C)neutrons can outnumber protons.
D)protons equal electrons.
A)protons can outnumber neutrons.
B)protons can outnumber electrons.
C)neutrons can outnumber protons.
D)protons equal electrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
From its atomic number of 10,you can predict that a neon atom:
A)is not chemically reactive.
B)has an unfilled outer shell.
C)has 10 neutrons.
D)can easily gain or lose electrons.
A)is not chemically reactive.
B)has an unfilled outer shell.
C)has 10 neutrons.
D)can easily gain or lose electrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following are found in the nucleus of an atom?
A)protons
B)neutrons
C)electrons
D)protons and neutrons
E)protons, neutrons, and electrons
A)protons
B)neutrons
C)electrons
D)protons and neutrons
E)protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
An atom will react with other atoms only until:
A)it has completely filled its outermost energy level.
B)it has less stability.
C)all of its inner orbitals have been filled.
D)it forms four covalent bonds
A)it has completely filled its outermost energy level.
B)it has less stability.
C)all of its inner orbitals have been filled.
D)it forms four covalent bonds

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
The naturally occurring helium atom is chemically inert because:
A)its outermost shell is filled with electrons.
B)its nucleus is filled with two neutrons.
C)it has the most protons that it could ever carry.
D)it has all of the shared electrons it could ever have.
A)its outermost shell is filled with electrons.
B)its nucleus is filled with two neutrons.
C)it has the most protons that it could ever carry.
D)it has all of the shared electrons it could ever have.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
You have a substance and begin a set of experiments in which you break it down into other substances through chemical reactions.After a few successive reactions,you discover a set of products that can't be broken down further,no matter what type of chemical reaction you attempt.These substances are:
A)protons.
B)elements.
C)neutrons.
D)electrons.
E)isotopes.
A)protons.
B)elements.
C)neutrons.
D)electrons.
E)isotopes.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following results from the making of a bond?
A)Atoms become more reactive.
B)Molecules are broken down.
C)Electrons are destroyed.
D)Atoms become more stable.
A)Atoms become more reactive.
B)Molecules are broken down.
C)Electrons are destroyed.
D)Atoms become more stable.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Isotopes are atoms of the same element that differ in their:
A)number of electrons.
B)number of neutrons.
C)number of protons.
D)ionic charge.
A)number of electrons.
B)number of neutrons.
C)number of protons.
D)ionic charge.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Atoms with eight electrons in their outer shells tend to:
A)form covalent bonds.
B)form ionic bonds.
C)be chemically reactive.
D)be stable and unreactive.
A)form covalent bonds.
B)form ionic bonds.
C)be chemically reactive.
D)be stable and unreactive.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
All the mass of an atom is considered to be in the:
A)protons only.
B)protons and neutrons.
C)electrons only.
D)protons, neutrons, and electrons.
A)protons only.
B)protons and neutrons.
C)electrons only.
D)protons, neutrons, and electrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
Two hydrogen atoms (atomic number 1)form a covalent bond.Which of the following is true?
A)Both hydrogen atoms now have two electrons in their outer shell.
B)Both hydrogen atoms now have two protons in their outer shell.
C)One hydrogen atom now has zero protons in its outer shell, and the other has two.
D)One hydrogen atom now has zero electrons in its outer shell, and the other has two.
E)Each hydrogen atom still has one electron in its outer shell.
A)Both hydrogen atoms now have two electrons in their outer shell.
B)Both hydrogen atoms now have two protons in their outer shell.
C)One hydrogen atom now has zero protons in its outer shell, and the other has two.
D)One hydrogen atom now has zero electrons in its outer shell, and the other has two.
E)Each hydrogen atom still has one electron in its outer shell.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Sodium chloride (NaCl)crystals (table salt)form as a result of:
A)covalent bonding.
B)hydrogen bonding.
C)being chemically stable.
D)the attraction of oppositely charged particles for each other.
A)covalent bonding.
B)hydrogen bonding.
C)being chemically stable.
D)the attraction of oppositely charged particles for each other.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
The ionic bond of sodium chloride is formed as a result of:
A)sodium and chlorine sharing electrons.
B)both sodium and chlorine losing electrons.
C)sodium gaining an electron from chlorine.
D)sodium giving up an electron to chlorine.
E)sodium giving up a proton to chlorine.
A)sodium and chlorine sharing electrons.
B)both sodium and chlorine losing electrons.
C)sodium gaining an electron from chlorine.
D)sodium giving up an electron to chlorine.
E)sodium giving up a proton to chlorine.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Molecules of water stick to each other because:
A)hydrogen bonds form between the hydrogen atom of one molecule and the oxygen atom of another molecule.
B)water molecules are nonpolar, and nonpolar molecules stick together.
C)hydrogen bonds form between the hydrogen atom of one molecule and a hydrogen atom of another molecule.
D)covalent bonds form between the hydrogen atom of one molecule and the oxygen atom of another molecule.
A)hydrogen bonds form between the hydrogen atom of one molecule and the oxygen atom of another molecule.
B)water molecules are nonpolar, and nonpolar molecules stick together.
C)hydrogen bonds form between the hydrogen atom of one molecule and a hydrogen atom of another molecule.
D)covalent bonds form between the hydrogen atom of one molecule and the oxygen atom of another molecule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
When sodium chloride dissolves in water,the sodium and chloride ions are pulled into solution by:
A)ionic bonds that form between the ions and the water molecules.
B)the attraction of the sodium ions to the negatively charged oxygen, and the attraction of the chloride ions to the two positively charged hydrogens of the water molecules.
C)the attraction of the sodium ions to the positively charged oxygen, and the attraction of the chloride ions to the two negatively charged hydrogens of the water molecules.
D)covalent bonds that form between the ions and the water molecules.
A)ionic bonds that form between the ions and the water molecules.
B)the attraction of the sodium ions to the negatively charged oxygen, and the attraction of the chloride ions to the two positively charged hydrogens of the water molecules.
C)the attraction of the sodium ions to the positively charged oxygen, and the attraction of the chloride ions to the two negatively charged hydrogens of the water molecules.
D)covalent bonds that form between the ions and the water molecules.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is true of chemical bonds?
A)Atoms can achieve a higher energy state and less stability by forming bonds.
B)Electrons are always shared.
C)Electrons can be shared or completely transferred.
D)Chemical bonds cannot occur between two identical atoms.
A)Atoms can achieve a higher energy state and less stability by forming bonds.
B)Electrons are always shared.
C)Electrons can be shared or completely transferred.
D)Chemical bonds cannot occur between two identical atoms.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
Water is a polar molecule because:
A)oxygen is more electronegative than hydrogen.
B)hydrogen has more neutrons than oxygen.
C)oxygen has more electrons than hydrogen.
D)oxygen has more neutrons than hydrogen.
E)hydrogen is more electronegative than oxygen.
A)oxygen is more electronegative than hydrogen.
B)hydrogen has more neutrons than oxygen.
C)oxygen has more electrons than hydrogen.
D)oxygen has more neutrons than hydrogen.
E)hydrogen is more electronegative than oxygen.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
The number of atoms coming out of a chemical reaction must equal the number of atoms going into a chemical reaction.This follows the principle of:
A)the law of conservation of energy.
B)chemical bonding.
C)atomic theory.
D)the law of conservation of mass.
A)the law of conservation of energy.
B)chemical bonding.
C)atomic theory.
D)the law of conservation of mass.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Oxygen has six electrons in its second outer shell,and hydrogen has one.With how many hydrogen atoms will oxygen form covalent bonds?
A)eight
B)one
C)two
D)six
E)three
A)eight
B)one
C)two
D)six
E)three

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
You mix sugar in water and stir until it's completely dissolved.In this system,the water is the ________,the sugar is the ________,and the end result is a ________.
A)solute; solution; solvent
B)solute; solvent; solution
C)solvent; solution; solute
D)solution; solvent; solute
E)solvent; solute; solution
A)solute; solution; solvent
B)solute; solvent; solution
C)solvent; solution; solute
D)solution; solvent; solute
E)solvent; solute; solution

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
An atom becomes an ion when:
A)it gains or loses neutrons.
B)it forms a covalent bond.
C)it gains or loses electrons.
D)hydrogen ions are shared.
E)it gains or loses protons.
A)it gains or loses neutrons.
B)it forms a covalent bond.
C)it gains or loses electrons.
D)hydrogen ions are shared.
E)it gains or loses protons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following would form the fewest covalent bonds?
A)neon (eight electrons in the second shell)
B)carbon (four electrons in the second shell)
C)hydrogen (one electron in the first shell)
D)oxygen (six electrons in the second shell)
A)neon (eight electrons in the second shell)
B)carbon (four electrons in the second shell)
C)hydrogen (one electron in the first shell)
D)oxygen (six electrons in the second shell)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
In what ways are hydrogen bonds and ionic bonds similar?
A)Both are based on attraction between atoms that carry differences in electrical charge.
B)Both involve an even sharing of electrons between atoms.
C)Both are based on attraction between two atoms where each carries a positive charge.
D)Both are based on repulsion between atoms that carry differences in electrical charge.
E)Both are based on attraction between two atoms where each carries a negative charge.
A)Both are based on attraction between atoms that carry differences in electrical charge.
B)Both involve an even sharing of electrons between atoms.
C)Both are based on attraction between two atoms where each carries a positive charge.
D)Both are based on repulsion between atoms that carry differences in electrical charge.
E)Both are based on attraction between two atoms where each carries a negative charge.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
Hydrogen bonds are very important in the functional shape of:
A)proteins.
B)sugars.
C)fats.
D)nucleic acids.
E)proteins and nucleic acids.
A)proteins.
B)sugars.
C)fats.
D)nucleic acids.
E)proteins and nucleic acids.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
Atoms form bonds to:
A)fill their outer shells with neutrons.
B)obtain an equal number of protons and electrons.
C)fill their outer shells with electrons.
D)fill their outer shells with protons.
E)obtain an equal number of protons and neutrons.
A)fill their outer shells with neutrons.
B)obtain an equal number of protons and electrons.
C)fill their outer shells with electrons.
D)fill their outer shells with protons.
E)obtain an equal number of protons and neutrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Hydrophobic molecules tend to be ________ by water.
A)repelled
B)absorbed
C)mixed
D)attracted
A)repelled
B)absorbed
C)mixed
D)attracted

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
What is the difference between an ionic and covalent bond?
A)In an ionic bond, one atom accepts electrons from the other; in a covalent bond, a pair of atoms share electrons.
B)In an ionic bond, one atom has more electronegativity than the other; in a covalent bond, the atoms have the same electronegativity.
C)Ionic bonding involves the inner electron shells; covalent bonding involves the valence electron shell.
D)Ionic bonds form between atoms of different elements; covalent bonds form between atoms of the same element.
A)In an ionic bond, one atom accepts electrons from the other; in a covalent bond, a pair of atoms share electrons.
B)In an ionic bond, one atom has more electronegativity than the other; in a covalent bond, the atoms have the same electronegativity.
C)Ionic bonding involves the inner electron shells; covalent bonding involves the valence electron shell.
D)Ionic bonds form between atoms of different elements; covalent bonds form between atoms of the same element.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
In a bottle of water,hydrogen bonding occurs between the hydrogen of one atom and a/an:
A)hydrogen atom in the same molecule.
B)oxygen atom in a different molecule.
C)oxygen atom in the same water molecule.
D)hydrogen atom in a different molecule.
A)hydrogen atom in the same molecule.
B)oxygen atom in a different molecule.
C)oxygen atom in the same water molecule.
D)hydrogen atom in a different molecule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
In hydrogen bonding,hydrogen nearly always pairs with:
A)another hydrogen.
B)carbon.
C)oxygen or nitrogen.
D)sodium or chlorine.
A)another hydrogen.
B)carbon.
C)oxygen or nitrogen.
D)sodium or chlorine.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Potassium has one electron in its fourth shell,and chlorine has seven electrons in its third shell.Which of the following is most likely to be true?
A)Chlorine will give an electron to potassium to form an ionic bond.
B)Potassium will give an electron to chlorine to form an ionic bond.
C)The two atoms will share the electron unequally in a polar covalent bond.
D)The two atoms will share an electron equally in a nonpolar covalent bond.
A)Chlorine will give an electron to potassium to form an ionic bond.
B)Potassium will give an electron to chlorine to form an ionic bond.
C)The two atoms will share the electron unequally in a polar covalent bond.
D)The two atoms will share an electron equally in a nonpolar covalent bond.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
An atom always contains the same number of protons as neutrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
The high specific heat and surface tension of water are a result of:
A)ionic bonds.
B)covalent bonds within the water molecules.
C)the size of water molecules.
D)hydrogen bonding between water molecules.
E)covalent bonds between water molecules.
A)ionic bonds.
B)covalent bonds within the water molecules.
C)the size of water molecules.
D)hydrogen bonding between water molecules.
E)covalent bonds between water molecules.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
The number of neutrons in the nucleus of an atom gives it a unique chemical nature.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
Anything that occupies space and has mass is energy.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Neutrons are negatively charged.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
The electrons of an atom contribute significantly to the mass of an atom.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
You shake up a bottle of vinegar and oil dressing to mix it each time you use it.The reason you need to do this is that:
A)oil is hydrophobic and won't dissolve in vinegar, so the oil and vinegar separate upon standing.
B)vinegar and oil are oppositely charged, and opposites attract.
C)fat molecules are too large to dissolve in water.
D)vinegar has an acidic pH and is neutralized when mixed with oil.
A)oil is hydrophobic and won't dissolve in vinegar, so the oil and vinegar separate upon standing.
B)vinegar and oil are oppositely charged, and opposites attract.
C)fat molecules are too large to dissolve in water.
D)vinegar has an acidic pH and is neutralized when mixed with oil.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Hydrogen bonds may form between oxygen of one water molecule and ________ of another water molecule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Water molecules are uncharged and ________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
An element can't be broken down into another form of pure matter.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Atoms are electrically neutral.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Acids release hydrogen ions into aqueous solutions.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
________ orbit around the nucleus of an atom.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
Ionic bonds occur through a sharing of electrons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Isotopes differ from each other in the number of protons that they possess.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
A single covalent chemical bond represents a sharing of ________ electrons between two atoms.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Buffering systems work to maintain pH within normal limits by:
A)adding hydrogen ions when conditions becomes too acidic.
B)adding hydroxide ions when conditions become too basic.
C)removing hydrogen ions when conditions become too acidic and adding hydrogen ions when conditions become too basic.
D)adding hydrogen ions when conditions become too acidic and removing hydrogen ions when conditions become too basic.
A)adding hydrogen ions when conditions becomes too acidic.
B)adding hydroxide ions when conditions become too basic.
C)removing hydrogen ions when conditions become too acidic and adding hydrogen ions when conditions become too basic.
D)adding hydrogen ions when conditions become too acidic and removing hydrogen ions when conditions become too basic.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
As an acid mixes in water:
A)the number of hydroxide ions will increase.
B)the number of hydrogen ions will increase.
C)the pH remains at 7.
D)it becomes buffered.
A)the number of hydroxide ions will increase.
B)the number of hydrogen ions will increase.
C)the pH remains at 7.
D)it becomes buffered.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Chemical reactions involve only the outermost electrons of an atom.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Which elements make up the majority of the human body?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Refer to the figure below, and then answer the question that follows.
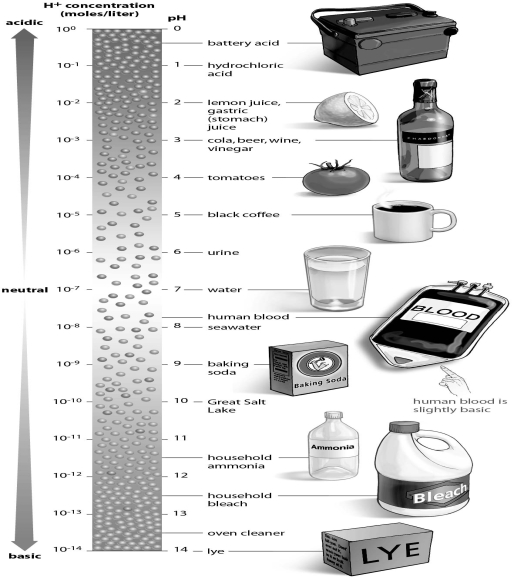
You are working in a chemistry lab,and your lab partner knocks over a beaker of hydrochloric acid.You alert your laboratory instructor,and he immediately pours another solution over the spill to neutralize the acid.Using the figure as a guide,what did your instructor pour onto the acid to neutralize it?
A)water
B)baking soda
C)lemon juice
D)coffee

You are working in a chemistry lab,and your lab partner knocks over a beaker of hydrochloric acid.You alert your laboratory instructor,and he immediately pours another solution over the spill to neutralize the acid.Using the figure as a guide,what did your instructor pour onto the acid to neutralize it?
A)water
B)baking soda
C)lemon juice
D)coffee

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
How are ions formed? Why do ionic compounds readily dissolve in water?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
A signal molecule will ________ to a receptor if the molecules' shapes match,similar to a key in a lock.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
What are the three most important subatomic particles in an atom called? Which one is involved in forming chemical bonds?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
What is chemical bonding? Explain the differences between covalent and ionic bonding.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Explain how a polar molecule,such as water,can have a difference in electrical charge but is also electrically neutral.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Refer to the figure below, and then answer the question that follows.
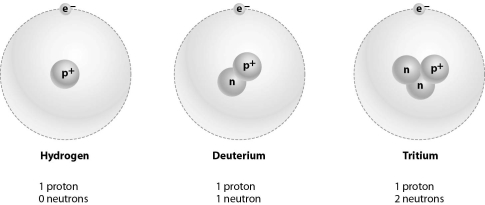
Hydrogen,deuterium,and tritium are considered the same element because:
A)their mass is about the same.
B)they can form ions easily.
C)they have the same number of protons.

Hydrogen,deuterium,and tritium are considered the same element because:
A)their mass is about the same.
B)they can form ions easily.
C)they have the same number of protons.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Refer to the figure below, and then answer the question that follows.
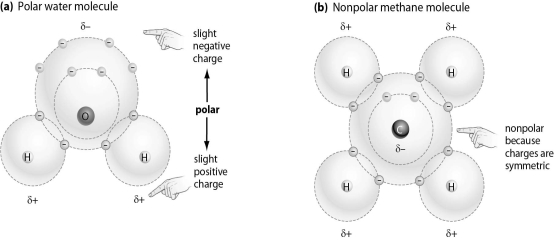
Which of the following molecules is most likely to bind to an ion,and why?
A)Molecule A, because it has electrical charges that will attract an ion
B)Molecule B, because it has four hydrogen atoms on the exterior of the molecule
C)Molecule A, because any molecule with oxygen is able to bind to an ion
D)Molecule B, because it has a carbon at in the center of the molecule

Which of the following molecules is most likely to bind to an ion,and why?
A)Molecule A, because it has electrical charges that will attract an ion
B)Molecule B, because it has four hydrogen atoms on the exterior of the molecule
C)Molecule A, because any molecule with oxygen is able to bind to an ion
D)Molecule B, because it has a carbon at in the center of the molecule

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Oil spills in the ocean are often treated with chemical dispersants.These materials are similar to detergents in that the molecules have both hydrophilic and hydrophobic portions.Based on this,predict what will happen when chemical dispersants are used to treat oil spills.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
You have been having trouble with acid indigestion recently.You buy some milk of magnesia,an antacid,from the drug store to relieve your indigestion.Milk of magnesia is a mixture of magnesium hydroxide in water.What makes milk of magnesia a good antacid? If you could chemically analyze your stomach fluids,what would you find before and after taking the antacid?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
It takes more energy to raise the temperature of water than of alcohol because water has a higher ________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Temperatures on the Earth are moderated by the presence of so much water on the planet.Using your understanding of water's temperature-moderating abilities,predict what would happen to temperatures in the tropical and temperate regions if the oceans were made of alcohol instead of water.(Hint: Water has a higher specific heat than alcohol.)

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
A(n)________ has a higher pH than a(n)________.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
Match between columns

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


