Deck 4: Proteins: Three-Dimensional Structure and Function
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/116
Play
Full screen (f)
Deck 4: Proteins: Three-Dimensional Structure and Function
1
Which statement is not true about the peptide bond?
A)The peptide bond has partial double-bond character.
B)The peptide bond is longer than the typical carbon-nitrogen bond.
C)Rotation is restricted about the peptide bond.
D)The carbonyl oxygen and the amide hydrogen are most often in a trans configuration with respect to one another.
A)The peptide bond has partial double-bond character.
B)The peptide bond is longer than the typical carbon-nitrogen bond.
C)Rotation is restricted about the peptide bond.
D)The carbonyl oxygen and the amide hydrogen are most often in a trans configuration with respect to one another.
The peptide bond is longer than the typical carbon-nitrogen bond.
2
Which technique is commonly used to determine the three-dimensional conformation of a protein?
A)Isoelectric focusing.
B)The Edman degradation.
C)SDS-PAGE.
D)X-ray crystallography.
A)Isoelectric focusing.
B)The Edman degradation.
C)SDS-PAGE.
D)X-ray crystallography.
X-ray crystallography.
3
Nearly all peptide bonds are in the trans configuration because
A)cis peptide bonds are weaker.
B)trans peptide bonds are stronger.
C)cis peptide bonds prevent R groups from interacting.
D)trans peptide bonds minimize steric hindrance of R groups.
A)cis peptide bonds are weaker.
B)trans peptide bonds are stronger.
C)cis peptide bonds prevent R groups from interacting.
D)trans peptide bonds minimize steric hindrance of R groups.
trans peptide bonds minimize steric hindrance of R groups.
4
What does it mean to say a protein is oligomeric?
A)In vivo it establishes an equilibrium between two or more active conformations.
B)It has more than fifty amino acids.
C)The active protein involves the association of two or more polypeptide chains.
D)The protein has multiple α-helices.
A)In vivo it establishes an equilibrium between two or more active conformations.
B)It has more than fifty amino acids.
C)The active protein involves the association of two or more polypeptide chains.
D)The protein has multiple α-helices.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
5
NMR is often used for the determination of the ________ of proteins.
A)molecular weight
B)isoelectric point
C)tertiary structure
D)pKa
A)molecular weight
B)isoelectric point
C)tertiary structure
D)pKa

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
6
Structural proteins that typically assemble into large cables or threads to provide mechanical support to cells or organisms are classified as ________ proteins.
A)fibrous
B)enzyme
C)globular
D)β-strand
A)fibrous
B)enzyme
C)globular
D)β-strand

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
7
In 1962 Kendrew and Perutz won a Nobel Prize for
A)solving the structure of collagen by NMR.
B)determining the structure of the α-helix.
C)performing the first sequencing of a protein.
D)determining the structures of myoglobin and hemoglobin by X-ray crystallography.
A)solving the structure of collagen by NMR.
B)determining the structure of the α-helix.
C)performing the first sequencing of a protein.
D)determining the structures of myoglobin and hemoglobin by X-ray crystallography.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
8
________ is a technique used to analyze the macromolecular structure of proteins in solution.
A)X-ray crystallography
B)SDS-PAGE
C)Affinity chromatography
D)NMR
A)X-ray crystallography
B)SDS-PAGE
C)Affinity chromatography
D)NMR

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
9
________ received a Nobel Prize in 1964 for determining the structure of vitamin B12.He/she also solved the structure of penicillin in 1947 and developed many techniques used in the study of large proteins.
A)Max Perutz
B)Linus Pauling
C)Rosalind Franklin
D)Dorothy Crowfoot
A)Max Perutz
B)Linus Pauling
C)Rosalind Franklin
D)Dorothy Crowfoot

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
10
Proteomics is
A)the study of large sets of proteins,such as the entire complement of proteins produced by a cell.
B)the manipulation of protein sequences to develop new proteins.
C)the copying of proteins to generate a lot of molecules from a small sample.
D)the specific study of the energy costs for protein synthesis.
E)the study of DNA/protein complexes.
A)the study of large sets of proteins,such as the entire complement of proteins produced by a cell.
B)the manipulation of protein sequences to develop new proteins.
C)the copying of proteins to generate a lot of molecules from a small sample.
D)the specific study of the energy costs for protein synthesis.
E)the study of DNA/protein complexes.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
11
To what level of structure do α-helices belong?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
A)Primary
B)Secondary
C)Tertiary
D)Quaternary

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
12
Which is a reasonable number of different polypeptides found in humans?
A)2000
B)20,000
C)2,000,000
D)2,000,000,000
A)2000
B)20,000
C)2,000,000
D)2,000,000,000

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
13
Which statement is false about the determination of protein structures?
A)NMR can generate sets of structures which may represent protein fluctuations.
B)The structures found by NMR and X-ray crystallography are usually very different.
C)It is often difficult to produce quality protein crystals for X-ray analysis.
D)NMR spectroscopy exposes protein solutions to a magnetic field.
A)NMR can generate sets of structures which may represent protein fluctuations.
B)The structures found by NMR and X-ray crystallography are usually very different.
C)It is often difficult to produce quality protein crystals for X-ray analysis.
D)NMR spectroscopy exposes protein solutions to a magnetic field.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
14
A change from one conformation of a molecule to another involves ________.
A)rotation about bonds only
B)breaking and reforming of covalent bonds
C)inversion about a center of symmetry
D)Any of the above
A)rotation about bonds only
B)breaking and reforming of covalent bonds
C)inversion about a center of symmetry
D)Any of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
15
The ________ is the single shape a protein adopts under physiological conditions.
A)minimal configuration
B)native conformation
C)primary structure
D)most stable enantiomer
A)minimal configuration
B)native conformation
C)primary structure
D)most stable enantiomer

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
16
Which is evidence that structures for proteins determined by X-ray crystallography represent the structures in solution?
A)Their similarity to structures determined by NMR.
B)The protein crystals are soluble in water.
C)The proteins must align in a regular pattern to form a crystal.
D)Only one conformation is ever possible for a protein so it is irrelevant whether the protein is in a crystal or in solution.
A)Their similarity to structures determined by NMR.
B)The protein crystals are soluble in water.
C)The proteins must align in a regular pattern to form a crystal.
D)Only one conformation is ever possible for a protein so it is irrelevant whether the protein is in a crystal or in solution.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
17
Which pair represents different conformations?
A)Cis-1,2-dichloroethene and trans-1,2-dichloroethene.
B)L-glycine and D-glycine.
C)Eclipsed ethane and staggered ethane.
D)Pentane and 2-methylbutane.
A)Cis-1,2-dichloroethene and trans-1,2-dichloroethene.
B)L-glycine and D-glycine.
C)Eclipsed ethane and staggered ethane.
D)Pentane and 2-methylbutane.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
18
In peptide bonds,the bonds between
A)C and N are shorter than typical C-N bonds.
B)C and N are longer than typical C-N bonds.
C)C and O are longer than typical C=O bonds.
D)C and O are shorter than typical C=O bonds.
E)Both A and C.
A)C and N are shorter than typical C-N bonds.
B)C and N are longer than typical C-N bonds.
C)C and O are longer than typical C=O bonds.
D)C and O are shorter than typical C=O bonds.
E)Both A and C.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement is false about a globular protein that performs its biological function as a single independent polypeptide chain?
A)Its tertiary structure is likely stabilized by the interactions of amino acid side chains in non-neighboring regions of the polypeptide chain.
B)It could contain α-helices that are stabilized by hydrogen bonding.
C)It likely has extensive quaternary structure to maintain its globular shape.
D)Non-covalent forces are the primary source of stability for the secondary and tertiary structure.
A)Its tertiary structure is likely stabilized by the interactions of amino acid side chains in non-neighboring regions of the polypeptide chain.
B)It could contain α-helices that are stabilized by hydrogen bonding.
C)It likely has extensive quaternary structure to maintain its globular shape.
D)Non-covalent forces are the primary source of stability for the secondary and tertiary structure.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
20
Computers are used to advance the understanding of three-dimensional protein structure by
A)determining the specific sequence of amino acids.
B)finding repeating subunit patterns.
C)calculating atomic positions from X-ray diffraction patterns.
D)collimating X-ray beams.
A)determining the specific sequence of amino acids.
B)finding repeating subunit patterns.
C)calculating atomic positions from X-ray diffraction patterns.
D)collimating X-ray beams.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
21
Proline is not often found in α-helices of proteins because it
A)has a small,uncharged side chain.
B)has a very bulky side chain.
C)lacks a hydrogen atom on its amide nitrogen.
D)interacts with adjacent amino acids.
A)has a small,uncharged side chain.
B)has a very bulky side chain.
C)lacks a hydrogen atom on its amide nitrogen.
D)interacts with adjacent amino acids.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement is NOT true about an α-helix?
A)It is usually right-handed.
B)It is a type of secondary structure.
C)It frequently contains proline residues.
D)It is stabilized by hydrogen bonding.
A)It is usually right-handed.
B)It is a type of secondary structure.
C)It frequently contains proline residues.
D)It is stabilized by hydrogen bonding.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
23
What would you expect about the formation of an α-helix for a segment of a protein chain that contains lysine approximately every fourth residue with all other residues being mostly hydrophobic?
A)Helix formation would be favored at low pH.
B)Helix formation would be favored at high pH.
C)Helix formation would be favored at neutral pH.
D)Helix formation would never occur regardless of pH.
A)Helix formation would be favored at low pH.
B)Helix formation would be favored at high pH.
C)Helix formation would be favored at neutral pH.
D)Helix formation would never occur regardless of pH.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
24
Ramachandran determined the "allowed" values of the phi and psi angles primarily by considering ________.
A)pKa values of the amino acids
B)the hydropathy of amino acids
C)steric hindrance
D)hydrogen bonding effects
A)pKa values of the amino acids
B)the hydropathy of amino acids
C)steric hindrance
D)hydrogen bonding effects

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
25
What is true about the rotation about bonds in a protein backbone?
A)The rotation is free about all bonds in the backbone,except for the bond between the nitrogen and the alpha carbon in proline residues.
B)The bond between the carbonyl carbon and nitrogen is restricted.Other bonds are free to rotate depending only on steric hindrance or the presence of proline residues.
C)All bonds in the backbone have restricted rotation and partial double-bond character.
D)The rotation is free only about the peptide bond.The other bonds are restricted by steric hindrance and the presence of proline residues.
A)The rotation is free about all bonds in the backbone,except for the bond between the nitrogen and the alpha carbon in proline residues.
B)The bond between the carbonyl carbon and nitrogen is restricted.Other bonds are free to rotate depending only on steric hindrance or the presence of proline residues.
C)All bonds in the backbone have restricted rotation and partial double-bond character.
D)The rotation is free only about the peptide bond.The other bonds are restricted by steric hindrance and the presence of proline residues.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
26
Which represents the backbone of a protein? Note: R = amino acid side chain
N = nitrogen
Cα = alpha carbon
C = carbonyl carbon
A)R1R2R3R4R5.
B)Repeating units of N-C.
C)Repeating units of N-Cα-C.
D)Repeating units of Cα-C.
N = nitrogen
Cα = alpha carbon
C = carbonyl carbon
A)R1R2R3R4R5.
B)Repeating units of N-C.
C)Repeating units of N-Cα-C.
D)Repeating units of Cα-C.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
27
The distance along a helix axis for one complete turn is called the ________.
A)axial distance
B)rise
C)screw length
D)pitch
A)axial distance
B)rise
C)screw length
D)pitch

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
28
An ideal α-helix has a pitch of 0.54 nm and a rise of 0.15 nm.What is the length along the helix axis for a segment of an ideal α-helix that contains 30 amino acids?
A)200 nm
B)4.50 nm
C)16.20 nm
D)55.5 nm
A)200 nm
B)4.50 nm
C)16.20 nm
D)55.5 nm

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
29
A helical wheel can be used to show
A)the pitch of a helix structure.
B)the amphipathic nature of a helix.
C)DNA binding.
D)pleated sheets.
A)the pitch of a helix structure.
B)the amphipathic nature of a helix.
C)DNA binding.
D)pleated sheets.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
30
What feature does a Ramachandran plot display?
A)Allowed angles of phi and psi for a polypeptide backbone.
B)Preferred amino acids in an α-helix.
C)The hydropathy of amino acids.
D)The variation of pH versus volume of base added during titration to determine the pKa.
A)Allowed angles of phi and psi for a polypeptide backbone.
B)Preferred amino acids in an α-helix.
C)The hydropathy of amino acids.
D)The variation of pH versus volume of base added during titration to determine the pKa.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
31
Which was a major scientific accomplishment by Linus Pauling?
A)Determined the α-helix structure in keratin.
B)Discovered β-strands and described their assembly into sheets.
C)Determined the three-dimensional structure of hemoglobin and myoglobin.
D)Determined the structure of penicillin.
A)Determined the α-helix structure in keratin.
B)Discovered β-strands and described their assembly into sheets.
C)Determined the three-dimensional structure of hemoglobin and myoglobin.
D)Determined the structure of penicillin.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
32
Which is true about the side chains of residues in an α-helix?
A)They extend above or below the pleats.
B)They extend radially outward from the helix axis.
C)They point toward the center of the helix.
D)They hydrogen bond extensively with each other.
A)They extend above or below the pleats.
B)They extend radially outward from the helix axis.
C)They point toward the center of the helix.
D)They hydrogen bond extensively with each other.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
33
The conformation of the backbone of a polypeptide is described completely by the angle(s)of rotation about which bond(s)?
A)The peptide bond only.
B)N-Cα only.
C)N-Cα,Cα-C and C-N bonds.
D)N-Cα and Cα-C bonds only.
A)The peptide bond only.
B)N-Cα only.
C)N-Cα,Cα-C and C-N bonds.
D)N-Cα and Cα-C bonds only.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
34
Which structure below indicates the proper hydrogen-bonding pattern between amino acids in an α-helix? (Dashed lines represent the hydrogen bonds. ) 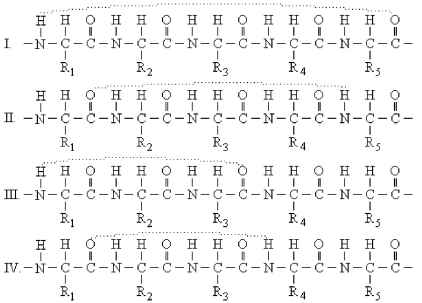
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
35
The trans configuration of most peptide groups is adopted in cells because it
A)is the only one available.
B)minimizes steric hindrance of R groups.
C)is favored in protein synthesis.
D)Both B and C.
E)All of the above.
A)is the only one available.
B)minimizes steric hindrance of R groups.
C)is favored in protein synthesis.
D)Both B and C.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
36
Proteins with alpha helix regions called leucine zippers are
A)often found in DNA binding proteins.
B)amphipathic helices.
C)part of pairs of helices that are wrapped around each other.
D)All of the above.
A)often found in DNA binding proteins.
B)amphipathic helices.
C)part of pairs of helices that are wrapped around each other.
D)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
37
Enzymes implicated in several human hereditary diseases are similar to ones that regulate flowering in Arabidopsis.These enzymes are
A)peptidyl cis isomerases.
B)proline isomerase.
C)prolyl cis/trans isomerase.
D)isomerase hydrolase.
A)peptidyl cis isomerases.
B)proline isomerase.
C)prolyl cis/trans isomerase.
D)isomerase hydrolase.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
38
The amino acid that destabilizes alpha-helical structures and is usually found at the ends of alpha helices is
A)glycine.
B)alanine.
C)asparagine.
D)glutamate.
A)glycine.
B)alanine.
C)asparagine.
D)glutamate.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
39
How many complete turns are there in an ideal α-helix that contains 15 amino acids and has a pitch of 0.54 nm and a rise of 0.15 nm?
A)1
B)4
C)8
D)59
A)1
B)4
C)8
D)59

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
40
Which is not true about β-sheets?
A)The side-chains of all amino acids point to the same side of the sheet.
B)The polypeptide chains in the sheet are nearly fully extended.
C)The range of allowed phi and psi angles is broader than for those in the α-helix.
D)In antiparallel sheets the hydrogen bonds between adjacent strands are nearly perpendicular to the backbones of the strands.
A)The side-chains of all amino acids point to the same side of the sheet.
B)The polypeptide chains in the sheet are nearly fully extended.
C)The range of allowed phi and psi angles is broader than for those in the α-helix.
D)In antiparallel sheets the hydrogen bonds between adjacent strands are nearly perpendicular to the backbones of the strands.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement is true about disulfide bonds and protein folding?
A)Disulfide bonds have no influence on protein folding since they only form after the protein adopts its native conformation.
B)Proteins occasionally adopt nonnative conformations and form improper disulfide bonds that can be reversed by the enzyme protein disulfide isomerase.
C)The folding of the native conformation is influenced more strongly by the formation of disulfide bonds than by the primary structure of a protein.
D)If a protein forms an improper disulfide bond it is irreversibly inactivated and must be targeted by the cell for degradation.
A)Disulfide bonds have no influence on protein folding since they only form after the protein adopts its native conformation.
B)Proteins occasionally adopt nonnative conformations and form improper disulfide bonds that can be reversed by the enzyme protein disulfide isomerase.
C)The folding of the native conformation is influenced more strongly by the formation of disulfide bonds than by the primary structure of a protein.
D)If a protein forms an improper disulfide bond it is irreversibly inactivated and must be targeted by the cell for degradation.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
42
Supersecondary structures that contain recognizable combinations of α-helices,β-strands and loops (e.g.the Greek Key)are called ________.
A)domains
B)folds
C)homologous regions
D)motifs
A)domains
B)folds
C)homologous regions
D)motifs

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
43
Loops and turns in proteins are
A)regions that let a polypeptide chain fold back on itself.
B)stretches of non-repeating three dimensional structures in proteins.
C)regions causing direction change in the polypeptide backbone.
D)All of the above.
A)regions that let a polypeptide chain fold back on itself.
B)stretches of non-repeating three dimensional structures in proteins.
C)regions causing direction change in the polypeptide backbone.
D)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
44
Tertiary structure of proteins describe
A)polypeptide folding.
B)bringing amino acids far apart in primary structure close together.
C)stabilizing protein structure by non-covalent interaction.
D)disulfide bridges.
E)All of the above.
A)polypeptide folding.
B)bringing amino acids far apart in primary structure close together.
C)stabilizing protein structure by non-covalent interaction.
D)disulfide bridges.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
45
Which factor does not help to explain why many proteins exhibit quaternary structure?
A)Oligomers are usually more stable than the free monomers.
B)Active sites can be formed when the protein chains associate.
C)The subunits always are able to maintain the same three-dimensional structure whether they are associated into an oligomer or not.
D)Increased efficiency by the sharing of the same subunit with the same function among different proteins.
A)Oligomers are usually more stable than the free monomers.
B)Active sites can be formed when the protein chains associate.
C)The subunits always are able to maintain the same three-dimensional structure whether they are associated into an oligomer or not.
D)Increased efficiency by the sharing of the same subunit with the same function among different proteins.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
46
A β-sandwich forms when
A)two hydrophobic sides of β-sheets interact.
B)two hydrophilic sides of β-sheets interact.
C)an α-helix separates two β-sheets.
D)two amphipathic α-helices interact.
A)two hydrophobic sides of β-sheets interact.
B)two hydrophilic sides of β-sheets interact.
C)an α-helix separates two β-sheets.
D)two amphipathic α-helices interact.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
47
Protein folding can be followed in the laboratory by measuring
A)viscosity.
B)electrophoretic mobility and gel filtration.
C)UV absorption and light rotation.
D)A,B,and C above.
E)A and C above.
A)viscosity.
B)electrophoretic mobility and gel filtration.
C)UV absorption and light rotation.
D)A,B,and C above.
E)A and C above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
48
In addition to self assembly,some proteins fold with the help of
A)energy stabilizers.
B)weak chemical interactions.
C)other proteins.
D)low entropy pickets.
E)All of the above.
A)energy stabilizers.
B)weak chemical interactions.
C)other proteins.
D)low entropy pickets.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
49
________ is used to estimate the molecular weight of oligomeric proteins,while ________ is used to determine molecular weight of each chain.
A)Melting point;SDS-gel electrophoresis
B)SDS-gel electrophoresis;gel-filtration chromatography
C)Acrylamide gel electrophoresis;isoelectric focusing
D)Gel-filtration chromatography;SDS-gel electrophoresis
E)SDS-gel electrophoresis;NMR
A)Melting point;SDS-gel electrophoresis
B)SDS-gel electrophoresis;gel-filtration chromatography
C)Acrylamide gel electrophoresis;isoelectric focusing
D)Gel-filtration chromatography;SDS-gel electrophoresis
E)SDS-gel electrophoresis;NMR

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
50
Interactomes describe interactions between
A)DNA and RNA.
B)RNA polymerase and RNA.
C)DNA polymerase and DNA.
D)Proteins with other proteins.
A)DNA and RNA.
B)RNA polymerase and RNA.
C)DNA polymerase and DNA.
D)Proteins with other proteins.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
51
A protein has a molecular weight of 5600 daltons;its subunits are about 1960 daltons.There are ________ protein chains per oligomer.
A)4
B)3
C)2
D)1
E)Cannot determine from the information given.
A)4
B)3
C)2
D)1
E)Cannot determine from the information given.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
52
Which demonstrates that the primary structure of a protein determines its tertiary structure?
A)How the disulfide bonds hold it in the correct shape.
B)Proteins can refold even when the amino acid sequence is changed.
C)Proteins refold when the amino acid sequence is the same as in the native conformation.
D)Chaotropic agents cannot denature the native conformation.
E)All of the above.
A)How the disulfide bonds hold it in the correct shape.
B)Proteins can refold even when the amino acid sequence is changed.
C)Proteins refold when the amino acid sequence is the same as in the native conformation.
D)Chaotropic agents cannot denature the native conformation.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
53
Protein subunits in a multisubunit protein are held to each other primarily by
A)covalent bonds.
B)hydrophobic interactions exclusively.
C)both strong and weak interactions.
D)hydrophobic and other weak interactions.
E)All of the above.
A)covalent bonds.
B)hydrophobic interactions exclusively.
C)both strong and weak interactions.
D)hydrophobic and other weak interactions.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
54
A tetrameric protein contains
A)four different subunits.
B)four identical subunits.
C)three subunits and one prosthetic group.
D)A or B only.
E)A,B or C.
A)four different subunits.
B)four identical subunits.
C)three subunits and one prosthetic group.
D)A or B only.
E)A,B or C.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
55
Protein X can bind to either protein A or protein B to form a complex.The association constants are 108 M-1 (X with A)and 106 M-1 (X with B).Which statement is true?
A)In the presence of excess X,it takes fewer molecules of A than B to generate a given amount of complex.
B)The dissociation constant of the complex XA is higher than that of complex XB.
C)B must be a larger protein than A.
D)The values of the association constants indicate that both complexes XA and XB are very unstable in small cells like E.coli.
A)In the presence of excess X,it takes fewer molecules of A than B to generate a given amount of complex.
B)The dissociation constant of the complex XA is higher than that of complex XB.
C)B must be a larger protein than A.
D)The values of the association constants indicate that both complexes XA and XB are very unstable in small cells like E.coli.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
56
The principle forces holding subunits of an oligomeric protein to each other are ________.
A)peptide bonds
B)hydrophobic interactions
C)covalent bonds
D)disulfide bonds
A)peptide bonds
B)hydrophobic interactions
C)covalent bonds
D)disulfide bonds

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
57
Many proteins have multiple subunits because
A)an active site is shared by different subunits.
B)they are more stable and flexible in movement.
C)different combinations can perform different functions.
D)All of the above.
E)A and C above.
A)an active site is shared by different subunits.
B)they are more stable and flexible in movement.
C)different combinations can perform different functions.
D)All of the above.
E)A and C above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
58
Which is not an example of an oligomeric protein?
A)Cytochrome c.
B)Potassium channel protein.
C)MS2 capsid protein.
D)Hemoglobin.
E)Bacterial photosynthetic reaction center.
A)Cytochrome c.
B)Potassium channel protein.
C)MS2 capsid protein.
D)Hemoglobin.
E)Bacterial photosynthetic reaction center.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
59
How many monomers are there in an oligomeric protein designated αβ2γ2?
A)2
B)3
C)4
D)5
E)Not given.
A)2
B)3
C)4
D)5
E)Not given.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
60
The Tm of a protein is temperature at which it
A)is completely denatured.
B)liquifies.
C)is half denatured.
D)resists denaturation.
A)is completely denatured.
B)liquifies.
C)is half denatured.
D)resists denaturation.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
61
Water can easily penetrate and even pass through the interior of a folded protein.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
62
Antibodies bind antigens at
A)light chains.
B)heavy chains.
C)hypervariable regions.
D)glycoprotein regions.
E)All of the above.
A)light chains.
B)heavy chains.
C)hypervariable regions.
D)glycoprotein regions.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
63
All proteins possess primary,secondary,tertiary and quaternary structure.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
64
Hemoglobin is
A)a tetramer of 4 myoglobin proteins.
B)a tetramer of four globin chains and one heme prosthetic group.
C)a dimer of subunits each with two distinct protein chains (alpha and beta).
D)a dimer of subunits each with two myoglobin proteins.
E)an erythrocyte.
A)a tetramer of 4 myoglobin proteins.
B)a tetramer of four globin chains and one heme prosthetic group.
C)a dimer of subunits each with two distinct protein chains (alpha and beta).
D)a dimer of subunits each with two myoglobin proteins.
E)an erythrocyte.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
65
α-helices easily form on the surface of water-soluble proteins due to the stabilizing effect of hydrogen bonding with water.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
66
Collagen is a protein made of a triple helix that is stabilized by
A)disulfide bridges.
B)intrachain hydrogen bonds.
C)interchain hydrogen bonds.
D)covalent bonds.
A)disulfide bridges.
B)intrachain hydrogen bonds.
C)interchain hydrogen bonds.
D)covalent bonds.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
67
NMR has the advantage of using proteins in solution,but it is difficult to determine the structures of very large proteins with this technique.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
68
Antibodies are suitable for diagnostic tests because
A)they can be made radioactive.
B)they can be readily purified.
C)they are found in very small quantities.
D)they bind very specifically to antigens.
E)they are found everywhere.
A)they can be made radioactive.
B)they can be readily purified.
C)they are found in very small quantities.
D)they bind very specifically to antigens.
E)they are found everywhere.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
69
The main property of myoglobin and hemoglobin that makes them an efficient system for oxygen delivery from lungs to muscles is
A)hydrophobicity.
B)different binding affinities for oxygen.
C)movement of the protein shapes.
D)cooperativity.
E)All of the above.
A)hydrophobicity.
B)different binding affinities for oxygen.
C)movement of the protein shapes.
D)cooperativity.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
70
Proteins segments which fold first can promote the folding of other sections of the protein into the native conformation by a process known as
A)renaturation.
B)stabilization.
C)hydrophobic interaction.
D)disulfide bridge formation.
E)cooperativity.
A)renaturation.
B)stabilization.
C)hydrophobic interaction.
D)disulfide bridge formation.
E)cooperativity.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
71
Which statement is false about the heme group?
A)When oxygen binds to heme,the iron ion is oxidized from Fe²+ to Fe ³+.
B)If exposed to air,a free heme group (not associated with hemoglobin)is readily oxidized converting Fe²+ to Fe ³+ and can no longer bind oxygen.
C)The heme group is tightly,but non-covalently,held in myoglobin molecule.
D)The chemical structure of the heme groups in myoglobin and hemoglobin are identical.
A)When oxygen binds to heme,the iron ion is oxidized from Fe²+ to Fe ³+.
B)If exposed to air,a free heme group (not associated with hemoglobin)is readily oxidized converting Fe²+ to Fe ³+ and can no longer bind oxygen.
C)The heme group is tightly,but non-covalently,held in myoglobin molecule.
D)The chemical structure of the heme groups in myoglobin and hemoglobin are identical.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
72
Proline exists in the cis configuration more frequently than any other amino acid.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
73
Conditions in the tissues which enhance the delivery of oxygen by hemoglobin are the presence of
A)carbon dioxide.
B)2,3 BPG.
C)protons.
D)All of the above.
E)A and B above.
A)carbon dioxide.
B)2,3 BPG.
C)protons.
D)All of the above.
E)A and B above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
74
All amino acids can readily interconvert between the cis and trans forms by rotation about the peptide bond.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
75
Cooperative binding of oxygen by hemoglobin
A)is induced by hemoglobin.
B)is a result of different affinities for oxygen by each subunit protein.
C)is induced by oxygenation.
D)is a result of interaction with myoglobin.
A)is induced by hemoglobin.
B)is a result of different affinities for oxygen by each subunit protein.
C)is induced by oxygenation.
D)is a result of interaction with myoglobin.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
76
Chaperones are proteins which
A)renature any denatured proteins.
B)help cells repair damage due to heat shock.
C)assist protein self assembly.
D)use ATP to fold proteins.
E)All of the above.
A)renature any denatured proteins.
B)help cells repair damage due to heat shock.
C)assist protein self assembly.
D)use ATP to fold proteins.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
77
β-strands are a type of secondary structure.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
78
A hyperbolic binding curve differs from a sigmoidal binding curve in that the hyperbolic curve
A)has a single equilibrium constant for oxygen binding.
B)binds more oxygen after the initial proteins first bind oxygen.
C)shows cooperativity.
D)binds up to four molecules of oxygen.
E)All of the above.
A)has a single equilibrium constant for oxygen binding.
B)binds more oxygen after the initial proteins first bind oxygen.
C)shows cooperativity.
D)binds up to four molecules of oxygen.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
79
Proteins with very high Tm values are generally stable at
A)room temperature (21°C).
B)50° to 60°C.
C)temperatures below 50°C.
D)temperatures above 60°C.
E)All of the above.
A)room temperature (21°C).
B)50° to 60°C.
C)temperatures below 50°C.
D)temperatures above 60°C.
E)All of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
80
Hydrophobic amino acid sequences in myoglobin are responsible for
A)covalent bonding to the heme prosthetic group.
B)the folding of the polypeptide chain.
C)the irreversible binding of oxygen.
D)A and B above.
A)covalent bonding to the heme prosthetic group.
B)the folding of the polypeptide chain.
C)the irreversible binding of oxygen.
D)A and B above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck


