Deck 2: The Chemical Context of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 2: The Chemical Context of Life
1
Calcium has an atomic number of 20 and an atomic mass of 40. Therefore, a calcium atom must have
A)20 protons.
B)40 electrons.
C)40 neutrons.
D)A and B only
E)A, B, and C
A)20 protons.
B)40 electrons.
C)40 neutrons.
D)A and B only
E)A, B, and C
A
2
Different atomic forms of an element contain the same number of protons but a different number of neutrons. What are these different atomic forms called?
A)ions
B)isotopes
C)neutronic atoms
D)isomers
E)radioactive atoms
A)ions
B)isotopes
C)neutronic atoms
D)isomers
E)radioactive atoms
B
3
The nucleus of a nitrogen atom contains 7 neutrons and 7 protons. Which of the following is a correct statement concerning nitrogen?
A)The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14.
B)The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7.
C)The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams.
D)The nitrogen atom has a mass number of 7 grams and an atomic number of 14.
E)The nitrogen atom has a mass number of 14 and an atomic mass of approximately 14 daltons.
A)The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14.
B)The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7.
C)The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams.
D)The nitrogen atom has a mass number of 7 grams and an atomic number of 14.
E)The nitrogen atom has a mass number of 14 and an atomic mass of approximately 14 daltons.
E
4
How do isotopes of the same element differ from each other?
A)number of protons
B)number of electrons
C)number of neutrons
D)valence electron distribution
E)amount of radioactivity
A)number of protons
B)number of electrons
C)number of neutrons
D)valence electron distribution
E)amount of radioactivity

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
Each element is unique and different from other elements because of the number of protons in the nuclei of its atoms. Which of the following indicates the number of protons in an atom's nucleus?
A)atomic mass
B)atomic weight
C)atomic number
D)mass weight
E)mass number
A)atomic mass
B)atomic weight
C)atomic number
D)mass weight
E)mass number

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
The atomic number of neon is 10. Therefore, which of the following is correct about an atom of neon?
A)It has 8 electrons in its outer electron shell.
B)It is inert.
C)It has an atomic mass of 10 daltons.
D)A and B only
E)A, B, and C are correct.
A)It has 8 electrons in its outer electron shell.
B)It is inert.
C)It has an atomic mass of 10 daltons.
D)A and B only
E)A, B, and C are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
The atomic number of carbon is 6. Carbon-14 is heavier than carbon-12 because the atomic nucleus of carbon-14 contains _____ neutrons.
A)6
B)7
C)8
D)12
E)14
A)6
B)7
C)8
D)12
E)14

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
H is a radioactive isotope of hydrogen. One difference between hydrogen-1 ( H)and hydrogen-3 ( H)is that hydrogen-3 has
A)one more neutron and one more proton than hydrogen-1.
B)one more proton and one more electron than hydrogen-1.
C)one more electron and one more neutron than hydrogen-1.
D)two more neutrons than hydrogen-1.
E)two more protons than hydrogen-1.
A)one more neutron and one more proton than hydrogen-1.
B)one more proton and one more electron than hydrogen-1.
C)one more electron and one more neutron than hydrogen-1.
D)two more neutrons than hydrogen-1.
E)two more protons than hydrogen-1.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is False?
A)Atoms of the various elements differ in their number of subatomic particles.
B)All atoms of a particular element have the same number of protons in their nuclei.
C)The neutrons and protons present in the nucleus of an atom are almost identical in mass; each has a mass of about 1 dalton.
D)An atom is the smallest unit of an element that still retains the properties of the element.
E)Protons and electrons are electrically charged particles. Protons have one unit of negative charge, and electrons have one unit of positive charge.
A)Atoms of the various elements differ in their number of subatomic particles.
B)All atoms of a particular element have the same number of protons in their nuclei.
C)The neutrons and protons present in the nucleus of an atom are almost identical in mass; each has a mass of about 1 dalton.
D)An atom is the smallest unit of an element that still retains the properties of the element.
E)Protons and electrons are electrically charged particles. Protons have one unit of negative charge, and electrons have one unit of positive charge.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Oxygen has an atomic number of 8 and a mass number of 16. Thus, the atomic mass of an oxygen atom is
A)exactly 8 grams.
B)exactly 8 daltons.
C)approximately 16 grams.
D)approximately 16 daltons.
E)24 amu (atomic mass units).
A)exactly 8 grams.
B)exactly 8 daltons.
C)approximately 16 grams.
D)approximately 16 daltons.
E)24 amu (atomic mass units).

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
Electrons exist only at fixed levels of potential energy. However, if an atom absorbs sufficient energy, a possible result is that
A)an electron may move to an electron shell farther out from the nucleus.
B)an electron may move to an electron shell closer to the nucleus.
C)the atom may become a radioactive isotope.
D)the atom would become a positively charged ion, or cation.
E)the atom would become a negatively charged ion, or anion.
A)an electron may move to an electron shell farther out from the nucleus.
B)an electron may move to an electron shell closer to the nucleus.
C)the atom may become a radioactive isotope.
D)the atom would become a positively charged ion, or cation.
E)the atom would become a negatively charged ion, or anion.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
An atom with an atomic number of 9 and a mass number of 19 would have an atomic mass of approximately
A)9 daltons.
B)9 grams.
C)10 daltons.
D)20 grams.
E)19 daltons.
A)9 daltons.
B)9 grams.
C)10 daltons.
D)20 grams.
E)19 daltons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
What is the approximate atomic mass of an atom with 16 neutrons, 15 protons, and 15 electrons?
A)15 daltons
B)16 daltons
C)30 daltons
D)31 daltons
E)46 daltons
A)15 daltons
B)16 daltons
C)30 daltons
D)31 daltons
E)46 daltons

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by humans and other vertebrates?
A)nitrogen
B)calcium
C)iodine
D)sodium
E)phosphorus
A)nitrogen
B)calcium
C)iodine
D)sodium
E)phosphorus

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Three or four of the following statements are True and correct. Which one, if any, is False? If all the statements are True, choose answer E.
A)Carbon, hydrogen, oxygen, and nitrogen make up approximately 96% of living matter.
B)The trace element iodine is required only in very small quantities by vertebrates.
C)Virtually all organisms require the same elements in the same quantities.
D)Iron is an example of an element needed by all organisms.
E)All of the other statements are True and correct.
A)Carbon, hydrogen, oxygen, and nitrogen make up approximately 96% of living matter.
B)The trace element iodine is required only in very small quantities by vertebrates.
C)Virtually all organisms require the same elements in the same quantities.
D)Iron is an example of an element needed by all organisms.
E)All of the other statements are True and correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following best describes the relationship between the atoms described below?
A)They contain 31 and 32 electrons, respectively.
B)They are both phosphorus cations.
C)They are both phosphorus anions.
D)They are both isotopes of phosphorus.
E)They contain 31 and 32 protons, respectively.
A)They contain 31 and 32 electrons, respectively.
B)They are both phosphorus cations.
C)They are both phosphorus anions.
D)They are both isotopes of phosphorus.
E)They contain 31 and 32 protons, respectively.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
The mass number of an element can be easily approximated by adding together the number of __________ in an atom of that element.
A)protons and neutrons
B)energy levels
C)protons and electrons
D)neutrons and electrons
E)isotopes
A)protons and neutrons
B)energy levels
C)protons and electrons
D)neutrons and electrons
E)isotopes

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
A)carbon, sodium, chlorine, nitrogen
B)carbon, sulfur, phosphorus, hydrogen
C)oxygen, hydrogen, calcium, sodium
D)carbon, hydrogen, nitrogen, oxygen
E)carbon, oxygen, sulfur, calcium
A)carbon, sodium, chlorine, nitrogen
B)carbon, sulfur, phosphorus, hydrogen
C)oxygen, hydrogen, calcium, sodium
D)carbon, hydrogen, nitrogen, oxygen
E)carbon, oxygen, sulfur, calcium

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
One difference between carbon-12 ( C)and carbon-14 ( C)is that carbon-14 has
A)two more protons than carbon-12.
B)two more electrons than carbon-12.
C)two more neutrons than carbon-12.
D)A and C only
E)B and C only
A)two more protons than carbon-12.
B)two more electrons than carbon-12.
C)two more neutrons than carbon-12.
D)A and C only
E)B and C only

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following best describes the relationship between the atoms described below?
A)They are isomers.
B)They are polymers.
C)They are isotopes.
D)They contain 1 and 3 protons, respectively.
E)They each contain 1 neutron.
A)They are isomers.
B)They are polymers.
C)They are isotopes.
D)They contain 1 and 3 protons, respectively.
E)They each contain 1 neutron.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Please refer to Figure 2.1 to answer the following questions.
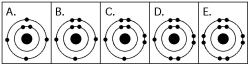
Figure 2.1
Which drawing depicts an atom that is inert or chemically unreactive?

Figure 2.1
Which drawing depicts an atom that is inert or chemically unreactive?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Which drawing depicts an atom with a valence of 3?

Figure 2.1
Which drawing depicts an atom with a valence of 3?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

-The atomic number of each atom is given to the left of each of the elements below. Which of the atoms has the same valence as carbon ( C)?
A)?nitrogen
B)?flourine
C)¹?neon
D)¹²magnesium
E)¹?silicon

-The atomic number of each atom is given to the left of each of the elements below. Which of the atoms has the same valence as carbon ( C)?
A)?nitrogen
B)?flourine
C)¹?neon
D)¹²magnesium
E)¹?silicon

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Which drawing depicts the electron configuration of neon (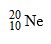 )?
)?

Figure 2.1
Which drawing depicts the electron configuration of neon (
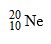 )?
)?
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
How many electrons does nitrogen have in its valence shell?
A)2
B)5
C)7
D)8
E)14

Figure 2.2
How many electrons does nitrogen have in its valence shell?
A)2
B)5
C)7
D)8
E)14

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
How many electrons does phosphorus have in its valence shell?
A)1
B)2
C)3
D)4
E)5

Figure 2.2
How many electrons does phosphorus have in its valence shell?
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Which drawing depicts the electron configuration of nitrogen (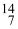 N)?
N)?

Figure 2.1
Which drawing depicts the electron configuration of nitrogen (
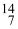 N)?
N)?
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
Based on electron configuration, which of these elements would exhibit chemical behavior most like that of oxygen?
A)carbon
B)hydrogen
C)nitrogen
D)sulfur
E)phosphorus

Figure 2.2
Based on electron configuration, which of these elements would exhibit chemical behavior most like that of oxygen?
A)carbon
B)hydrogen
C)nitrogen
D)sulfur
E)phosphorus

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
How many electrons would be expected in the outermost electron shell of an atom with atomic number 12?
A)1
B)2
C)4
D)6
E)8

Figure 2.2
How many electrons would be expected in the outermost electron shell of an atom with atomic number 12?
A)1
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
How many electrons does an atom of sulfur have in its valence shell?
A)4
B)6
C)8
D)16
E)32

Figure 2.2
How many electrons does an atom of sulfur have in its valence shell?
A)4
B)6
C)8
D)16
E)32

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
What is the valence of an atom with six electrons in its outer electron shell?
A)1
B)2
C)3
D)4
E)5

Figure 2.2
What is the valence of an atom with six electrons in its outer electron shell?
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Atoms whose outer electron shells contain eight electrons tend to
A)form ionic bonds in aqueous solutions.
B)form covalent bonds in aqueous solutions.
C)be stable and chemically nonreactive, or inert.
D)be unstable and chemically very reactive.
E)be isotopes and very radioactive.

Figure 2.1
Atoms whose outer electron shells contain eight electrons tend to
A)form ionic bonds in aqueous solutions.
B)form covalent bonds in aqueous solutions.
C)be stable and chemically nonreactive, or inert.
D)be unstable and chemically very reactive.
E)be isotopes and very radioactive.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
A)1
B)3
C)5
D)7
E)9

Figure 2.2
Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
A)1
B)3
C)5
D)7
E)9

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
From its atomic number of 15, it is possible to predict that the phosphorus atom has
A)15 neutrons.
B)15 protons.
C)15 electrons.
D)8 electrons in its outermost electron shell.
E)B and C only
A)15 neutrons.
B)15 protons.
C)15 electrons.
D)8 electrons in its outermost electron shell.
E)B and C only

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Which drawing depicts an atom with a valence of 2?

Figure 2.1
Which drawing depicts an atom with a valence of 2?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Which drawing depicts the electron configuration of oxygen ( O)?
O)?

Figure 2.1
Which drawing depicts the electron configuration of oxygen (
 O)?
O)?
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
What is the maximum number of electrons in a 2p orbital of an atom?
A)1
B)2
C)3
D)4
E)5

Figure 2.2
What is the maximum number of electrons in a 2p orbital of an atom?
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
What is the maximum number of electrons in the 1s orbital of an atom?
A)1
B)2
C)3
D)4
E)5

Figure 2.2
What is the maximum number of electrons in the 1s orbital of an atom?
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
Please refer to Figure 2.1 to answer the following questions.

Figure 2.1
Which drawing is of an atom with the atomic number of 6?

Figure 2.1
Which drawing is of an atom with the atomic number of 6?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.

Figure 2.2
How many neutrons are present in the nucleus of a phosphorus atom?
A)8
B)15
C)16
D)31
E)46

Figure 2.2
How many neutrons are present in the nucleus of a phosphorus atom?
A)8
B)15
C)16
D)31
E)46

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following explains most specifically the attraction of water molecules to one another?
A)nonpolar covalent bond
B)polar covalent bond
C)ionic bond
D)hydrogen bond
E)hydrophobic interaction
A)nonpolar covalent bond
B)polar covalent bond
C)ionic bond
D)hydrogen bond
E)hydrophobic interaction

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
The ionic bond of sodium chloride is formed when
A)chlorine gains an electron from sodium.
B)sodium and chlorine share an electron pair.
C)sodium and chlorine both lose electrons from their outer valence shells.
D)sodium gains an electron from chlorine.
E)chlorine gains a proton from sodium.
A)chlorine gains an electron from sodium.
B)sodium and chlorine share an electron pair.
C)sodium and chlorine both lose electrons from their outer valence shells.
D)sodium gains an electron from chlorine.
E)chlorine gains a proton from sodium.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
What is the difference between covalent bonds and ionic bonds?
A)Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of electrons between atoms.
B)Covalent bonds involve the sharing of neutrons between atoms, and ionic bonds involve the sharing of electrons between atoms.
C)Covalent bonds involve the sharing of electrons between atoms, and ionic bonds involve the electrical attraction between atoms.
D)Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of neutrons between atoms.
E)Covalent bonds involve the transfer of electrons between atoms, and ionic bonds involve the sharing of neutrons between atoms.
A)Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of electrons between atoms.
B)Covalent bonds involve the sharing of neutrons between atoms, and ionic bonds involve the sharing of electrons between atoms.
C)Covalent bonds involve the sharing of electrons between atoms, and ionic bonds involve the electrical attraction between atoms.
D)Covalent bonds involve the sharing of protons between atoms, and ionic bonds involve the sharing of neutrons between atoms.
E)Covalent bonds involve the transfer of electrons between atoms, and ionic bonds involve the sharing of neutrons between atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
A van der Waals interaction is the weak attraction between
A)the electrons of one molecule and the electrons of a nearby molecule.
B)the nucleus of one molecule and the electrons of a nearby molecule.
C)a polar molecule and a nearby nonpolar molecule.
D)a polar molecule and a nearby molecule that is also polar.
E)a nonpolar molecule and a nearby molecule that is also nonpolar.
A)the electrons of one molecule and the electrons of a nearby molecule.
B)the nucleus of one molecule and the electrons of a nearby molecule.
C)a polar molecule and a nearby nonpolar molecule.
D)a polar molecule and a nearby molecule that is also polar.
E)a nonpolar molecule and a nearby molecule that is also nonpolar.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
A covalent chemical bond is one in which
A)electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B)protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C)outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms.
D)outer-shell electrons of one atom are transferred to the inner electron shells of another atom.
E)the inner-shell electrons of one atom are transferred to the outer shell of another atom.
A)electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B)protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C)outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms.
D)outer-shell electrons of one atom are transferred to the inner electron shells of another atom.
E)the inner-shell electrons of one atom are transferred to the outer shell of another atom.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
If an atom of sulfur (atomic number 16)were allowed to react with atoms of hydrogen (atomic number 1), which of the molecules below would be formed?
A)S- H
B)H- S -H
C)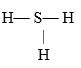
D)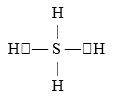
E)H=S=H
A)S- H
B)H- S -H
C)
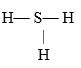
D)

E)H=S=H

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Van der Waals interactions result when
A)hybrid orbitals overlap.
B)electrons are not symmetrically distributed in a molecule.
C)molecules held by ionic bonds react with water.
D)two polar covalent bonds react.
E)a hydrogen atom loses an electron.
A)hybrid orbitals overlap.
B)electrons are not symmetrically distributed in a molecule.
C)molecules held by ionic bonds react with water.
D)two polar covalent bonds react.
E)a hydrogen atom loses an electron.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
In ammonium chloride salt (NH₄Cl)the anion is a single chloride ion, Cl⁻. What is the cation of NH4Cl?
A)N, with a charge of +3
B)H, with a charge of +1
C)H₂ with a charge of +4
D)NH₄ with a charge of +1
E)NH₄ with a charge of +4
A)N, with a charge of +3
B)H, with a charge of +1
C)H₂ with a charge of +4
D)NH₄ with a charge of +1
E)NH₄ with a charge of +4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is not considered to be a weak molecular interaction?
A)a covalent bond
B)a van der Waals interaction
C)an ionic bond in the presence of water
D)a hydrogen bond
E)A and B only
A)a covalent bond
B)a van der Waals interaction
C)an ionic bond in the presence of water
D)a hydrogen bond
E)A and B only

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
What results from an unequal sharing of electrons between atoms?
A)a nonpolar covalent bond
B)a polar covalent bond
C)an ionic bond
D)a hydrogen bond
E)a hydrophobic interaction
A)a nonpolar covalent bond
B)a polar covalent bond
C)an ionic bond
D)a hydrogen bond
E)a hydrophobic interaction

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
A molecule of carbon dioxide (CO₂)is formed when one atom of carbon (atomic number 6)is covalently bonded with two atoms of oxygen (atomic number 8). What is the total number of electrons that must be shared between the carbon atom and the oxygen atoms in order to complete the outer electron shell of all three atoms?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
The following questions refer to Figure 2.3.
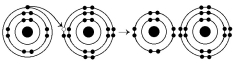
Figure 2.3
What results from the chemical reaction illustrated in Figure 2.3?
A)a cation with a net charge of +1
B)a cation with a net charge of -1
C)an anion with a net charge of +1
D)an anion with a net charge of -1
E)A and D

Figure 2.3
What results from the chemical reaction illustrated in Figure 2.3?
A)a cation with a net charge of +1
B)a cation with a net charge of -1
C)an anion with a net charge of +1
D)an anion with a net charge of -1
E)A and D

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
A covalent bond is likely to be polar when
A)one of the atoms sharing electrons is much more electronegative than the other atom.
B)the two atoms sharing electrons are equally electronegative.
C)the two atoms sharing electrons are of the same element.
D)it is between two atoms that are both very strong electron acceptors.
E)the two atoms sharing electrons are different elements.
A)one of the atoms sharing electrons is much more electronegative than the other atom.
B)the two atoms sharing electrons are equally electronegative.
C)the two atoms sharing electrons are of the same element.
D)it is between two atoms that are both very strong electron acceptors.
E)the two atoms sharing electrons are different elements.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
The following questions refer to Figure 2.3.

Figure 2.3
What is the atomic number of the cation formed in the reaction illustrated in Figure 2.3?
A)1
B)8
C)10
D)11
E)16

Figure 2.3
What is the atomic number of the cation formed in the reaction illustrated in Figure 2.3?
A)1
B)8
C)10
D)11
E)16

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following molecules contains the strongest polar covalent bond?
A)H₂
B)O₂
C)CO₂
D)H₂O
E)CH₄
A)H₂
B)O₂
C)CO₂
D)H₂O
E)CH₄

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
When two atoms are equally electronegative, they will interact to form
A)equal numbers of isotopes.
B)ions.
C)polar covalent bonds.
D)nonpolar covalent bonds.
E)ionic bonds.
A)equal numbers of isotopes.
B)ions.
C)polar covalent bonds.
D)nonpolar covalent bonds.
E)ionic bonds.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
The atomic number of chlorine is 17. The atomic number of magnesium is 12. What is the formula for magnesium chloride?
A)MgCl
B)MgCl₂
C)Mg₂Cl
D)Mg₂Cl₂
E)MgCl₃
A)MgCl
B)MgCl₂
C)Mg₂Cl
D)Mg₂Cl₂
E)MgCl₃

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Nitrogen (N)is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH₃)?
A)Each hydrogen atom has a partial positive charge.
B)The nitrogen atom has a strong positive charge.
C)Each hydrogen atom has a slight negative charge.
D)The nitrogen atom has a partial positive charge.
E)There are covalent bonds between the hydrogen atoms.
A)Each hydrogen atom has a partial positive charge.
B)The nitrogen atom has a strong positive charge.
C)Each hydrogen atom has a slight negative charge.
D)The nitrogen atom has a partial positive charge.
E)There are covalent bonds between the hydrogen atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
What is the maximum number of covalent bonds an element with atomic number 8 can make with hydrogen?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following results from a transfer of electron(s)between atoms?
A)nonpolar covalent bond
B)polar covalent bond
C)ionic bond
D)hydrogen bond
E)hydrophobic interaction
A)nonpolar covalent bond
B)polar covalent bond
C)ionic bond
D)hydrogen bond
E)hydrophobic interaction

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is True for this reaction? 3 H2 + N2 ↔ 2 NH3
A)The reaction is nonreversible.
B)Hydrogen and nitrogen are the reactants of the reverse reaction.
C)Hydrogen and nitrogen are the products of the forward reaction.
D)Ammonia is being formed and decomposed.
E)Hydrogen and nitrogen are being decomposed.
A)The reaction is nonreversible.
B)Hydrogen and nitrogen are the reactants of the reverse reaction.
C)Hydrogen and nitrogen are the products of the forward reaction.
D)Ammonia is being formed and decomposed.
E)Hydrogen and nitrogen are being decomposed.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
In the term trace element, the modifier trace means
A)the element is required in very small amounts.
B)the element can be used as a label to trace atoms through an organism's metabolism.
C)the element is very rare on Earth.
D)the element enhances health but is not essential for the organism's long-term survival.
E)the element passes rapidly through the organism.
A)the element is required in very small amounts.
B)the element can be used as a label to trace atoms through an organism's metabolism.
C)the element is very rare on Earth.
D)the element enhances health but is not essential for the organism's long-term survival.
E)the element passes rapidly through the organism.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Refer to the following figure to answer the following questions.
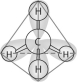
Which of the following pairs of atoms would be most likely to form a covalent bond?
A)
B)
C)
D)
E)

Which of the following pairs of atoms would be most likely to form a covalent bond?
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Refer to the following figure to answer the following questions.
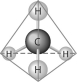
The molecule shown here is the simplest of organic compounds. It is called
A)a carbohydrate.
B)carbon dioxide.
C)methane.
D)carbonic hydrate.
E)methyl carbonate.

The molecule shown here is the simplest of organic compounds. It is called
A)a carbohydrate.
B)carbon dioxide.
C)methane.
D)carbonic hydrate.
E)methyl carbonate.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound:
A)HS
B)HS₂
C)H₂S
D)H₃S₂
E)H₄S
A)HS
B)HS₂
C)H₂S
D)H₃S₂
E)H₄S

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
Refer to the following figure to answer the following questions.

In the methane molecule shown here, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electrons in these bonds are said to have
A)double orbitals.
B)tetrahedral orbitals.
C)complex orbitals.
D)hybrid orbitals.
E)reduced orbitals.

In the methane molecule shown here, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electrons in these bonds are said to have
A)double orbitals.
B)tetrahedral orbitals.
C)complex orbitals.
D)hybrid orbitals.
E)reduced orbitals.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
Which statement is True of all atoms that are anions?
A)The atom has more electrons than protons.
B)The atom has more protons than electrons.
C)The atom has fewer protons than does a neutral atom of the same element.
D)The atom has more neutrons than protons.
E)The net charge is 12.
A)The atom has more electrons than protons.
B)The atom has more protons than electrons.
C)The atom has fewer protons than does a neutral atom of the same element.
D)The atom has more neutrons than protons.
E)The net charge is 12.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following would be regarded as compounds?
A)H₂
B)H₂O
C)O₂
D)CH₄
E)B and D, but not A and C
A)H₂
B)H₂O
C)O₂
D)CH₄
E)B and D, but not A and C

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
Refer to the following figure to answer the following questions.

The molecule shown here could be described in chemical symbols as
A)CH₄.
B)H₂O.
C)C₂H₃.
D)C₄H₄.
E)CH₂O.

The molecule shown here could be described in chemical symbols as
A)CH₄.
B)H₂O.
C)C₂H₃.
D)C₄H₄.
E)CH₂O.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
The reactivity of an atom arises from
A)the average distance of the outermost electron shell from the nucleus.
B)the existence of unpaired electrons in the valence shell.
C)the sum of the potential energies of all the electron shells.
D)the potential energy of the valence shell.
E)the energy difference between the s and p orbitals.
A)the average distance of the outermost electron shell from the nucleus.
B)the existence of unpaired electrons in the valence shell.
C)the sum of the potential energies of all the electron shells.
D)the potential energy of the valence shell.
E)the energy difference between the s and p orbitals.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Sometimes atoms form molecules by sharing two pairs of valence electrons. When this occurs, the atoms are said to be joined by
A)a double covalent bond.
B)an electronegative bond.
C)a hydrogen bond.
D)a protonic bond.
E)a complex bond.
A)a double covalent bond.
B)an electronegative bond.
C)a hydrogen bond.
D)a protonic bond.
E)a complex bond.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
The hybrid orbitals in a molecule of methane are oriented
A)toward the corners of a tetrahedron centered on the carbon atom.
B)toward the corners of a cube centered on the carbon atom.
C)toward the corners of a triangle centered on the carbon atom.
D)toward the corners of a rectangle centered on the carbon atom.
E)toward the edges of an oval centered on the carbon atom.
A)toward the corners of a tetrahedron centered on the carbon atom.
B)toward the corners of a cube centered on the carbon atom.
C)toward the corners of a triangle centered on the carbon atom.
D)toward the corners of a rectangle centered on the carbon atom.
E)toward the edges of an oval centered on the carbon atom.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
Refer to the following figure to answer the following questions.

Which of the following pairs of atoms would be most likely to form an ionic bond?
A)
B)
C)
D)
E)

Which of the following pairs of atoms would be most likely to form an ionic bond?
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following describes any reaction that has reached chemical equilibrium?
A)The concentration of the reactants equals the concentration of the products.
B)The rate of the forward reaction is equal to the rate of the reverse reaction.
C)All of the reactants have been converted to the products of the reaction.
D)All of the products have been converted to the reactants of the reaction.
E)Both the forward and the reverse reactions have stopped with no net effect on the concentration of the reactants and the products.
A)The concentration of the reactants equals the concentration of the products.
B)The rate of the forward reaction is equal to the rate of the reverse reaction.
C)All of the reactants have been converted to the products of the reaction.
D)All of the products have been converted to the reactants of the reaction.
E)Both the forward and the reverse reactions have stopped with no net effect on the concentration of the reactants and the products.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following best describes chemical equilibrium?
A)Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B)Concentrations of products are higher than the concentrations of the reactants.
C)Forward and reverse reactions have stopped so that the concentration of the reactants equals the concentration of the products.
D)Reactions stop only when all reactants have been converted to products.
E)There are equal concentrations of reactants and products, and the reactions have stopped.
A)Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B)Concentrations of products are higher than the concentrations of the reactants.
C)Forward and reverse reactions have stopped so that the concentration of the reactants equals the concentration of the products.
D)Reactions stop only when all reactants have been converted to products.
E)There are equal concentrations of reactants and products, and the reactions have stopped.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Refer to the following figure to answer the following questions.

Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A)
B)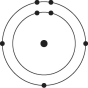
C)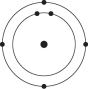
D)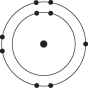
E)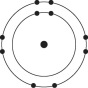

Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Refer to the following figure to answer the following questions.

Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A)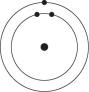
B)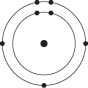
C)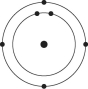
D)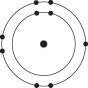
E)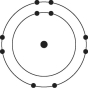

Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
A group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual behavior. They have turned to you for advice. Which of the following compounds is most likely to mimic the effects of the hormone?
A)a compound with the same number of carbon atoms as the hormone
B)a compound with the same molecular mass (measured in daltons)as the hormone
C)a compound with the same three-dimensional shape as part of the hormone
D)a compound with the same number of orbital electrons as the hormone
E)a compound with the same number of hydrogen and nitrogen atoms as the hormone
A)a compound with the same number of carbon atoms as the hormone
B)a compound with the same molecular mass (measured in daltons)as the hormone
C)a compound with the same three-dimensional shape as part of the hormone
D)a compound with the same number of orbital electrons as the hormone
E)a compound with the same number of hydrogen and nitrogen atoms as the hormone

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Compared with 31P, the radioactive isotope ³²P has
A)a different atomic number.
B)one more neutron.
C)one more proton.
D)one more electron.
E)a different charge.
A)a different atomic number.
B)one more neutron.
C)one more proton.
D)one more electron.
E)a different charge.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Atoms can be represented by simply listing the number of protons, neutrons, and electrons for example, 2p⁺; 2n⁰; 2e⁻ for helium. Which one of the following lists represents the 18O isotope of oxygen?
A)6p⁺; 8n⁰; 6e⁻
B)8p⁺; 10n⁰; 8e⁻
C)9p⁺; 9n⁰; 9e⁻
D)7p⁺; 2n⁰; 9e⁻
E)10p⁺; 8n⁰; 9e⁻
A)6p⁺; 8n⁰; 6e⁻
B)8p⁺; 10n⁰; 8e⁻
C)9p⁺; 9n⁰; 9e⁻
D)7p⁺; 2n⁰; 9e⁻
E)10p⁺; 8n⁰; 9e⁻

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


