Deck 4: Carbon and the Molecular Diversity of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 4: Carbon and the Molecular Diversity of Life
1
Which of the following people synthesized an organic compound, acetic acid, from inorganic substances that had been prepared directly from pure elements?
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
D
2
Which of the following people used this apparatus to study formation of organic compounds?
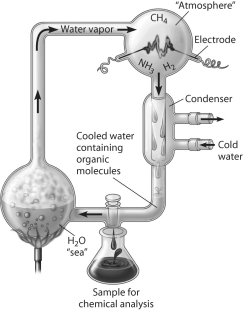
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
A
3
One of the following people set up a closed system to mimic Earth's early atmosphere and discharged electrical sparks through it. A variety of organic compounds common in organisms were formed. Who did this?
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
A
4
Organic chemistry is a science based on the study of
A)functional groups.
B)vital forces interacting with matter.
C)carbon compounds.
D)water and its interaction with other kinds of molecules.
E)inorganic compounds.
A)functional groups.
B)vital forces interacting with matter.
C)carbon compounds.
D)water and its interaction with other kinds of molecules.
E)inorganic compounds.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
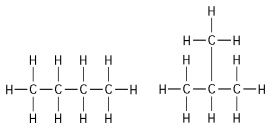
The two molecules shown in Figure 4.1 are best described as
A)optical isomers.
B)radioactive isotopes.
C)structural isomers.
D)nonradioactive isotopes.
E)geometric isomers.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
How many structural isomers are possible for a substance having the molecular formula C₄H₁₀?
A)1
B)2
C)4
D)3
E)11
A)1
B)2
C)4
D)3
E)11

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following people's synthesis of this compound from inorganic starting materials provided evidence against vitalism?
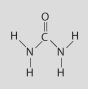
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following people's synthesis of this compound from inorganic starting materials provided evidence against vitalism?
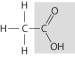
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
How many electron pairs does carbon share in order to complete its valence shell?
A)1
B)2
C)3
D)4
E)8
A)1
B)2
C)3
D)4
E)8

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
One of the following people was the first to suggest that organic compounds, those found in living organisms, were distinctly different from inorganic compounds found in the nonliving world. Though this suggestion is now known to be incorrect, it stimulated important research into organic compounds. Who suggested this?
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
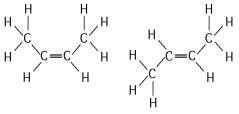
The two molecules shown in Figure 4.3 are best described as
A)enantiomers.
B)radioactive isotopes.
C)structural isomers.
D)nonisotopic isomers.
E)geometric isomers.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Why are hydrocarbons insoluble in water?
A)The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B)The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C)They are hydrophilic.
D)They exhibit considerable molecular complexity and diversity.
E)They are lighter than water.
A)The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B)The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C)They are hydrophilic.
D)They exhibit considerable molecular complexity and diversity.
E)They are lighter than water.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements best describes the carbon atoms present in all organic molecules?
A)They were incorporated into organic molecules by plants.
B)They were processed into sugars through photosynthesis.
C)They are ultimately derived from carbon dioxide.
D)Only A and C are correct.
E)A, B, and C are correct.
A)They were incorporated into organic molecules by plants.
B)They were processed into sugars through photosynthesis.
C)They are ultimately derived from carbon dioxide.
D)Only A and C are correct.
E)A, B, and C are correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
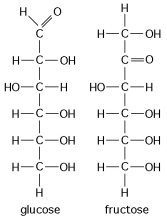
Shown here in Figure 4.2 are the structures of glucose and fructose. These two molecules differ in the
A)number of carbon, hydrogen, and oxygen atoms.
B)types of carbon, hydrogen, and oxygen atoms.
C)arrangement of carbon, hydrogen, and oxygen atoms.
D)number of oxygen atoms joined to carbon atoms by double covalent bonds.
E)answers A, B, and C

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
The experimental approach taken in current biological investigations presumes that
A)simple organic compounds can be synthesized in the laboratory from inorganic precursors, but complex organic compounds like carbohydrates and proteins can only be synthesized by living organisms.
B)a life force ultimately controls the activities of living organisms and this life force cannot be studied by physical or chemical methods.
C)although a life force, or vitalism, exists in living organisms, this life force cannot be studied by physical or chemical methods.
D)living organisms are composed of the same elements present in nonliving things, plus a few special trace elements found only in living organisms or their products.
E)living organisms can be understood in terms of the same physical and chemical laws that can be used to explain all natural phenomena.
A)simple organic compounds can be synthesized in the laboratory from inorganic precursors, but complex organic compounds like carbohydrates and proteins can only be synthesized by living organisms.
B)a life force ultimately controls the activities of living organisms and this life force cannot be studied by physical or chemical methods.
C)although a life force, or vitalism, exists in living organisms, this life force cannot be studied by physical or chemical methods.
D)living organisms are composed of the same elements present in nonliving things, plus a few special trace elements found only in living organisms or their products.
E)living organisms can be understood in terms of the same physical and chemical laws that can be used to explain all natural phenomena.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Early 19th-century scientists believed that living organisms differed from nonliving things as a result of possessing a "life force" that could create organic molecules from inorganic matter. The term given to this belief is
A)organic synthesis.
B)vitalism.
C)mechanism.
D)organic evolution.
E)inorganic synthesis.
A)organic synthesis.
B)vitalism.
C)mechanism.
D)organic evolution.
E)inorganic synthesis.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements correctly describes geometric isomers?
A)They have variations in arrangement around a double bond.
B)They have an asymmetric carbon that makes them mirror images.
C)They have the same chemical properties.
D)They have different molecular formulas.
E)Their atoms and bonds are arranged in different sequences.
A)They have variations in arrangement around a double bond.
B)They have an asymmetric carbon that makes them mirror images.
C)They have the same chemical properties.
D)They have different molecular formulas.
E)Their atoms and bonds are arranged in different sequences.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Shown here in Figure 4.2 are the structures of glucose and fructose. These two molecules are
A)geometric isotopes.
B)enantiomers.
C)geometric isomers.
D)structural isomers.
E)nonisotopic isomers.
A)geometric isotopes.
B)enantiomers.
C)geometric isomers.
D)structural isomers.
E)nonisotopic isomers.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
A carbon atom is most likely to form what kind of bond(s)with other atoms?
A)ionic
B)hydrogen
C)covalent
D)A and B only
E)A, B, and C
A)ionic
B)hydrogen
C)covalent
D)A and B only
E)A, B, and C

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following people was the first to synthesize an organic compound, urea, from inorganic starting materials?
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé
A)Stanley Miller
B)Jakob Berzelius
C)Friedrich Wohler
D)Hermann Kolbe
E)August Kekulé

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Which is the best description of a carbonyl group?
A)an oxygen joined to a carbon by a single covalent bond
B)a nitrogen and two hydrogens joined to a carbon by covalent bonds
C)a carbon joined to two hydrogens by single covalent bonds
D)a sulfur and a hydrogen joined to a carbon by covalent bonds
E)a carbon atom joined to an oxygen by a double covalent bond
A)an oxygen joined to a carbon by a single covalent bond
B)a nitrogen and two hydrogens joined to a carbon by covalent bonds
C)a carbon joined to two hydrogens by single covalent bonds
D)a sulfur and a hydrogen joined to a carbon by covalent bonds
E)a carbon atom joined to an oxygen by a double covalent bond

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following contains nitrogen in addition to carbon, oxygen, and hydrogen?
A)an alcohol such as ethanol
B)a monosaccharide such as glucose
C)a steroid such as testosterone
D)an amino acid such as glycine
E)a hydrocarbon such as benzene
A)an alcohol such as ethanol
B)a monosaccharide such as glucose
C)a steroid such as testosterone
D)an amino acid such as glycine
E)a hydrocarbon such as benzene

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Research indicates that Albuterol, a drug used to relax bronchial muscles, improving airflow and thus offering relief from asthma, consists only of one enantiomer, the R-form. Why is it important for this drug to consist of only one enantiomeric form, rather than a mixture of enantiomers?
A)Different enantiomers may have different or opposite physiological effects.
B)It is impossible to synthesize mixtures of enantiomers.
C)It is much less expensive to synthesize one enantiomer at a time.
D)Albuterol is an example of a compound for which only one enantiomer exists.
E)Only the R-form of Albuterol has been studied; until more information is available, physicians prefer to use the pure R-form.
A)Different enantiomers may have different or opposite physiological effects.
B)It is impossible to synthesize mixtures of enantiomers.
C)It is much less expensive to synthesize one enantiomer at a time.
D)Albuterol is an example of a compound for which only one enantiomer exists.
E)Only the R-form of Albuterol has been studied; until more information is available, physicians prefer to use the pure R-form.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
The following questions refer to the structures shown in Figure 4.5.
A.
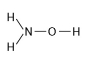
B.
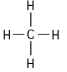
C.
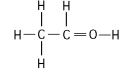
D.
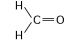
E.
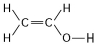
-In which of the structures are the atoms bonded by ionic bonds?
A)A
B)B
C)C
D)C, D, and E only
E)none of the structures
A.

B.

C.

D.

E.

-In which of the structures are the atoms bonded by ionic bonds?
A)A
B)B
C)C
D)C, D, and E only
E)none of the structures

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Which functional groups can act as acids?
A)amine and sulfhydryl
B)carbonyl and carboxyl
C)carboxyl and phosphate
D)hydroxyl and aldehyde
E)ketone and amino
A)amine and sulfhydryl
B)carbonyl and carboxyl
C)carboxyl and phosphate
D)hydroxyl and aldehyde
E)ketone and amino

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Which two functional groups are always found in amino acids?
A)ketone and aldehyde
B)carbonyl and carboxyl
C)carboxyl and amino
D)phosphate and sulfhydryl
E)hydroxyl and aldehyde
A)ketone and aldehyde
B)carbonyl and carboxyl
C)carboxyl and amino
D)phosphate and sulfhydryl
E)hydroxyl and aldehyde

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Three or four of the pairs of structures shown below depict enantiomers (enantiomeric forms)of the same molecule. Which pair, if any, are NOT enantiomers of a single molecule? If each of the pairs depicts enantiomers, choose answer F.
A)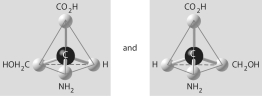
B)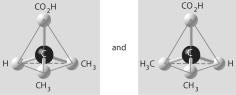
C)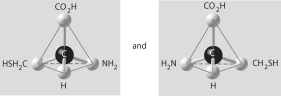
D)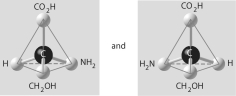
E)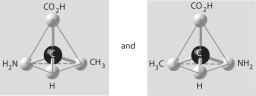
F)Both illustrations in each of the other answer choices depict enantiomers of the same molecule.
A)

B)

C)

D)

E)

F)Both illustrations in each of the other answer choices depict enantiomers of the same molecule.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Three or four of the following illustrations depict different structural isomers of the organic compound with molecular formula C₆H₁₄. For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted. Which one, if any, is NOT a structural isomer of this compound?
A)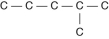
B)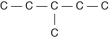
C)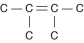
D)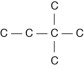
E)Each of the illustrations in the other answer choices depicts a structural isomer of the compound with molecular formula C₆H₁₄.
A)
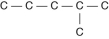
B)

C)
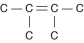
D)

E)Each of the illustrations in the other answer choices depicts a structural isomer of the compound with molecular formula C₆H₁₄.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Research indicates that Ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that
A)have identical three-dimensional shapes.
B)are mirror images of one another.
C)lack an asymmetric carbon.
D)differ in the location of their double bonds.
E)differ in their electrical charge.
A)have identical three-dimensional shapes.
B)are mirror images of one another.
C)lack an asymmetric carbon.
D)differ in the location of their double bonds.
E)differ in their electrical charge.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
A carbon skeleton is covalently bonded to both an amino group and a carboxyl group. When placed in water it
A)would function only as an acid because of the carboxyl group.
B)would function only as a base because of the amino group.
C)would function as neither an acid nor a base.
D)would function as both an acid and a base.
E)is impossible to determine how it would function.
A)would function only as an acid because of the carboxyl group.
B)would function only as a base because of the amino group.
C)would function as neither an acid nor a base.
D)would function as both an acid and a base.
E)is impossible to determine how it would function.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
The following questions refer to the structures shown in Figure 4.5.
A.

B.

C.

D.

E.

-Which of the structures is an impossible covalently bonded molecule?
A)A
B)B
C)C
D)D
E)E
A.

B.

C.

D.

E.

-Which of the structures is an impossible covalently bonded molecule?
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Thalidomide and L-dopa, shown below, are examples of pharmaceutical drugs that occur as enantiomers, or molecules that 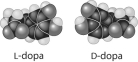
A)have identical three-dimensional shapes.
B)are mirror images of one another.
C)lack an asymmetric carbon.
D)differ in the location of their double bonds.
E)differ in their electrical charge.

A)have identical three-dimensional shapes.
B)are mirror images of one another.
C)lack an asymmetric carbon.
D)differ in the location of their double bonds.
E)differ in their electrical charge.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the pairs of molecular structures shown below depict enantiomers (enantiomeric forms)of the same molecule?
A)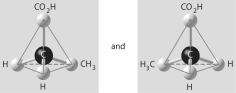
B)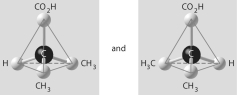
C)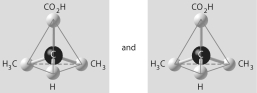
D)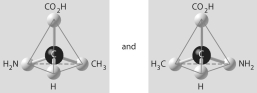
E)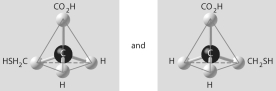
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a False statement concerning amino groups?
A)They are basic in pH.
B)They are found in amino acids.
C)They contain nitrogen.
D)They are nonpolar.
E)They are components of urea.
A)They are basic in pH.
B)They are found in amino acids.
C)They contain nitrogen.
D)They are nonpolar.
E)They are components of urea.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Amino acids are acids because they always possess which functional group?
A)amino
B)carbonyl
C)carboxyl
D)sulfhydryl
E)aldehyde
A)amino
B)carbonyl
C)carboxyl
D)sulfhydryl
E)aldehyde

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Figure 4.4 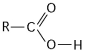
What is the name of the functional group shown in Figure 4.4?
A)carbonyl
B)ketone
C)aldehyde
D)carboxyl
E)hydroxyl

What is the name of the functional group shown in Figure 4.4?
A)carbonyl
B)ketone
C)aldehyde
D)carboxyl
E)hydroxyl

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
A chemist wishes to make an organic molecule less acidic. Which of the following functional groups should be added to the molecule in order to do so?
A)carboxyl
B)sulfhydryl
C)hydroxyl
D)amino
E)phosphate
A)carboxyl
B)sulfhydryl
C)hydroxyl
D)amino
E)phosphate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the pairs of molecular structures shown below do NOT depict enantiomers (enantiomeric forms)of the same molecule?
A)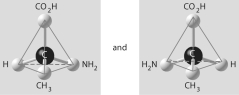
B)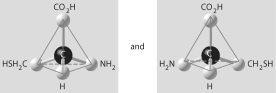
C)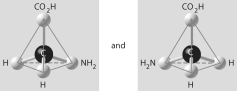
D)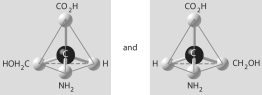
E)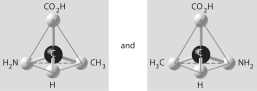
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
The following questions refer to the structures shown in Figure 4.5.
A.

B.

C.

D.

E.

-Which of the structures contain(s)a carboxyl functional group?
A)A
B)B
C)C
D)C and E
E)none of the structures
A.

B.

C.

D.

E.

-Which of the structures contain(s)a carboxyl functional group?
A)A
B)B
C)C
D)C and E
E)none of the structures

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
A compound contains hydroxyl groups as its predominant functional group. Which of the following statements is True concerning this compound?
A)It lacks an asymmetric carbon, and it is probably a fat or lipid.
B)It should dissolve in water.
C)It should dissolve in a nonpolar solvent.
D)It won't form hydrogen bonds with water.
E)It is hydrophobic.
A)It lacks an asymmetric carbon, and it is probably a fat or lipid.
B)It should dissolve in water.
C)It should dissolve in a nonpolar solvent.
D)It won't form hydrogen bonds with water.
E)It is hydrophobic.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
The following questions refer to the molecules shown in Figure 4.7.
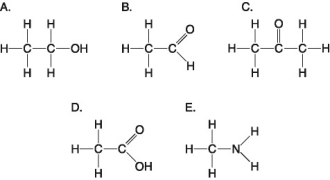
Which molecule can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?

Which molecule can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
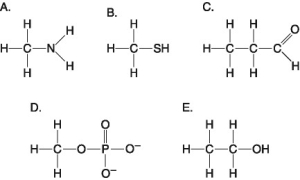
The following questions refer to the functional groups shown in Figure 4.6.
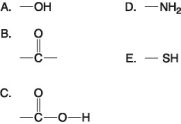
Which is a basic functional group that can accept H+ and become positively charged?

Which is a basic functional group that can accept H+ and become positively charged?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
The following questions refer to the molecules shown in Figure 4.7.

Which molecule contains a carboxyl group?

Which molecule contains a carboxyl group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
The following questions refer to the molecules shown in Figure 4.7.

Which molecule is an alcohol?

Which molecule is an alcohol?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
The following questions refer to the functional groups shown in Figure 4.6.

Which is an acidic functional group that can dissociate and release H+ into a solution?

Which is an acidic functional group that can dissociate and release H+ into a solution?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
The following questions refer to the molecules shown in Figure 4.8.
Which molecule contains a sulfhydryl functional group?

Which molecule contains a sulfhydryl functional group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
The following questions refer to the functional groups shown in Figure 4.6.

Which is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?

Which is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
The following questions refer to the molecules shown in Figure 4.8.

Which molecule is an organic phosphate?

Which molecule is an organic phosphate?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
The following questions refer to the functional groups shown in Figure 4.6.

Which is an amino functional group?

Which is an amino functional group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
The following questions refer to the functional groups shown in Figure 4.6.

Which is a carbonyl functional group?

Which is a carbonyl functional group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
The following questions refer to the molecules shown in Figure 4.8.

Which molecule can function as a base?

Which molecule can function as a base?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
The following questions refer to the molecules shown in Figure 4.7.

Which molecule has a carbonyl functional group in the form of a ketone?

Which molecule has a carbonyl functional group in the form of a ketone?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
The following questions refer to the molecules shown in Figure 4.7.

Which molecule is water soluble because it has a hydroxyl functional group?

Which molecule is water soluble because it has a hydroxyl functional group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
The following questions refer to the molecules shown in Figure 4.8.

Which molecule functions to transfer energy between organic molecules?

Which molecule functions to transfer energy between organic molecules?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
The following questions refer to the molecules shown in Figure 4.7.
A.
B.
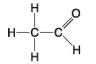
C.
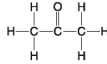
D.
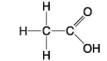
E.
-Which molecules contain a carbonyl group?
A)A and B
B)B and C
C)C and D
D)D and E
E)E and A
A.

B.

C.

D.

E.

-Which molecules contain a carbonyl group?
A)A and B
B)B and C
C)C and D
D)D and E
E)E and A

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
The following questions refer to the molecules shown in Figure 4.7.

Which molecule has a carbonyl functional group in the form of an aldehyde?

Which molecule has a carbonyl functional group in the form of an aldehyde?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
The following questions refer to the molecules shown in Figure 4.8.

Which molecule is a thiol?

Which molecule is a thiol?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
The following questions refer to the molecules shown in Figure 4.8.

Which molecule contains an amino functional group, but is not an amino acid?

Which molecule contains an amino functional group, but is not an amino acid?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
The following questions refer to the functional groups shown in Figure 4.6.

Which is a carboxyl functional group?

Which is a carboxyl functional group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
The following questions refer to the functional groups shown in Figure 4.6.

Which is a hydroxyl functional group?

Which is a hydroxyl functional group?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
The following questions refer to the molecules shown in Figure 4.8.
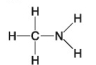
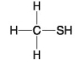
A.
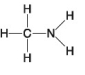
B.
C.
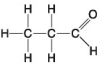
D.
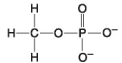
E.
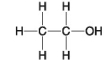
-Which of the following hydrocarbons has a double bond in its carbon skeleton?
A)C?H?
B)C?H?
C)CH?
D)C?H?
E)C?H?
A.

B.

C.

D.

E.

-Which of the following hydrocarbons has a double bond in its carbon skeleton?
A)C?H?
B)C?H?
C)CH?
D)C?H?
E)C?H?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
The following questions refer to the molecules shown in Figure 4.8.
A.

B.

C.

D.

E.

-Organic chemistry is currently defined as
A)the study of compounds made only by living cells.
B)the study of carbon compounds.
C)the study of vital forces.
D)the study of natural (as opposed to synthetic)compounds.
E)the study of hydrocarbons.
A.

B.

C.

D.

E.

-Organic chemistry is currently defined as
A)the study of compounds made only by living cells.
B)the study of carbon compounds.
C)the study of vital forces.
D)the study of natural (as opposed to synthetic)compounds.
E)the study of hydrocarbons.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Which action could produce a carbonyl group?
A)the replacement of the OH of a carboxyl group with hydrogen
B)the addition of a thiol to a hydroxyl
C)the addition of a hydroxyl to a phosphate
D)the replacement of the nitrogen of an amine with oxygen
E)the addition of a sulfhydryl to a carboxyl
A)the replacement of the OH of a carboxyl group with hydrogen
B)the addition of a thiol to a hydroxyl
C)the addition of a hydroxyl to a phosphate
D)the replacement of the nitrogen of an amine with oxygen
E)the addition of a sulfhydryl to a carboxyl

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Which functional group is not present in this molecule?
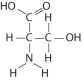
A)carboxyl
B)sulfhydryl
C)hydroxyl
D)amino

A)carboxyl
B)sulfhydryl
C)hydroxyl
D)amino

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
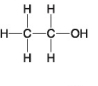
Choose the term that correctly describes the relationship between these two sugar molecules: 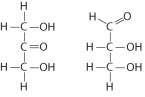
A)structural isomers
B)geometric isomers
C)enantiomers
D)isotopes

A)structural isomers
B)geometric isomers
C)enantiomers
D)isotopes

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
The following questions refer to the molecules shown in Figure 4.8.
A.

B.

C.

D.

E.

-Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s)do these molecules differ from each other?
A)Testosterone and estradiol are structural isomers but have the same molecular formula.
B)Testosterone and estradiol are geometric isomers but have the same molecular formula.
C)Testosterone and estradiol have different functional groups attached to the same carbon skeleton.
D)Testosterone and estradiol have distinctly different chemical structures, with one including four fused rings of carbon atoms, while the other has three rings.
E)Testosterone and estradiol are enantiomers of the same organic molecule.
A.

B.

C.

D.

E.

-Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s)do these molecules differ from each other?
A)Testosterone and estradiol are structural isomers but have the same molecular formula.
B)Testosterone and estradiol are geometric isomers but have the same molecular formula.
C)Testosterone and estradiol have different functional groups attached to the same carbon skeleton.
D)Testosterone and estradiol have distinctly different chemical structures, with one including four fused rings of carbon atoms, while the other has three rings.
E)Testosterone and estradiol are enantiomers of the same organic molecule.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
The following questions refer to the molecules shown in Figure 4.8.
A.

B.

C.

D.

E.

-Testosterone and estradiol are
A)nucleic acids.
B)carbohydrates.
C)proteins.
D)phospholipids.
E)steroids.
A.

B.

C.

D.

E.

-Testosterone and estradiol are
A)nucleic acids.
B)carbohydrates.
C)proteins.
D)phospholipids.
E)steroids.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Which chemical group is most likely to be responsible for an organic molecule behaving as a base?
A)hydroxyl
B)carbonyl
C)carboxyl
D)amino
E)phosphate
A)hydroxyl
B)carbonyl
C)carboxyl
D)amino
E)phosphate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


