Deck 3: Life Has a Unique Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/49
Play
Full screen (f)
Deck 3: Life Has a Unique Chemistry
1
Which of the following would dissolve most easily in water?
A)Fat
B)Salt
C)Oil
D)Butter
A)Fat
B)Salt
C)Oil
D)Butter
B
2
Which description of Ca²⁺ is most accurate?
A)A positive ion that has lost 2 electrons.
B)A positive ion that has gained 2 protons.
C)An isotope with 2 extra neutrons.
D)An atom that has gained 2 electrons.
A)A positive ion that has lost 2 electrons.
B)A positive ion that has gained 2 protons.
C)An isotope with 2 extra neutrons.
D)An atom that has gained 2 electrons.
A
3
Sweating is a good example of which of water's properties?
A)It is cohesive.
B)It is a good solvent.
C)It has a high heat of vaporization.
D)It has a high specific heat.
A)It is cohesive.
B)It is a good solvent.
C)It has a high heat of vaporization.
D)It has a high specific heat.
C
4
Which collection of elements are the most common in living organisms?
A)Oxygen, carbon, hydrogen, and nitrogen
B)Oxygen, sulfur, nitrogen, and carbon
C)Carbon, hydrogen, neon, and nitrogen
D)Oxygen, hydrogen, neon, and sulfur
A)Oxygen, carbon, hydrogen, and nitrogen
B)Oxygen, sulfur, nitrogen, and carbon
C)Carbon, hydrogen, neon, and nitrogen
D)Oxygen, hydrogen, neon, and sulfur

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
5
What is a nonpolar molecule?
A)The atoms share the electrons equally
B)Ions that become neutral from bonding
C)Water is a nonpolar molecule
D)The atoms bond by neutrons instead of electrons
A)The atoms share the electrons equally
B)Ions that become neutral from bonding
C)Water is a nonpolar molecule
D)The atoms bond by neutrons instead of electrons

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
6
Water's high specific heat makes it:
A)a good temperature buffer.
B)a good solvent.
C)liquid at room temperature.
D)easy to boil.
A)a good temperature buffer.
B)a good solvent.
C)liquid at room temperature.
D)easy to boil.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
7
What is the difference between Carbon-12 and Carbon-14?
A)Carbon-14 is older.
B)Carbon-14 has more neutrons.
C)Carbon-14 has more protons.
D)Carbon-14 has a half-life of 14 years.
A)Carbon-14 is older.
B)Carbon-14 has more neutrons.
C)Carbon-14 has more protons.
D)Carbon-14 has a half-life of 14 years.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
8
Select the most accurate description of an atom.
A)A nucleus of neutrons orbited by protons.
B)A nucleus of neutrons and protons orbited by electrons.
C)A nucleus of electrons orbited by neutrons and protons.
D)Protons orbiting around electrons.
A)A nucleus of neutrons orbited by protons.
B)A nucleus of neutrons and protons orbited by electrons.
C)A nucleus of electrons orbited by neutrons and protons.
D)Protons orbiting around electrons.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
9
The atomic number equals:
A)the number of electrons
B)the number of protons
C)the mass of the protons
D)the number of neutrons
A)the number of electrons
B)the number of protons
C)the mass of the protons
D)the number of neutrons

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
10
How can one tell how many electrons are in the outer energy level of an element?
A)Atomic mass
B)Atomic number
C)Roman numeral above each column in the periodic table
D)Subtract the atomic number from the atomic mass
A)Atomic mass
B)Atomic number
C)Roman numeral above each column in the periodic table
D)Subtract the atomic number from the atomic mass

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
11
What is the name of the bond shown here? 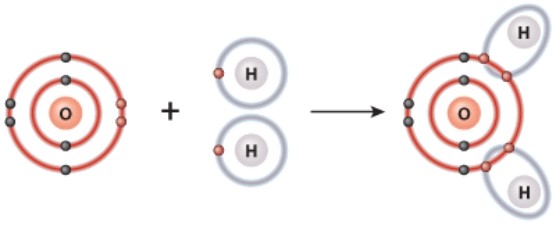
A)Hydrogen
B)Polar covalent
C)Ionic
D)Van der Waals forces

A)Hydrogen
B)Polar covalent
C)Ionic
D)Van der Waals forces

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is considered a base?
A)Ammonia
B)Lemon juice
C)Vinegar
D)Stomach acid
A)Ammonia
B)Lemon juice
C)Vinegar
D)Stomach acid

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following have approximately the same mass?
A)Protons and proteins
B)Neutrons and nuclei
C)Protons and neutrons
D)Protons and electrons
A)Protons and proteins
B)Neutrons and nuclei
C)Protons and neutrons
D)Protons and electrons

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
14
The bond holding NaCl together is a(n):
A)covalent bond.
B)ionic bond.
C)hydrogen bond.
D)salt bond.
A)covalent bond.
B)ionic bond.
C)hydrogen bond.
D)salt bond.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
15
Which bond is the weakest?
A)Peptide
B)Ionic
C)Covalent
D)Hydrogen
A)Peptide
B)Ionic
C)Covalent
D)Hydrogen

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
16
A change in which ion concentration would severely affect heart rate and the nervous system?
A)Bicarbonate
B)Sodium
C)Potassium
D)Chloride
A)Bicarbonate
B)Sodium
C)Potassium
D)Chloride

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
17
What makes an acid different from a base?
A)Acids contain more hydrogen ions than hydroxide ions.
B)Acids have a pH of 7 or greater.
C)Acids taste sweet.
D)Acids have equal amounts of hydrogen and hydroxide ions.
A)Acids contain more hydrogen ions than hydroxide ions.
B)Acids have a pH of 7 or greater.
C)Acids taste sweet.
D)Acids have equal amounts of hydrogen and hydroxide ions.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
18
Which element is the most common in humans?
A)Chloride
B)Phosphorus
C)Hydrogen
D)Sulfur
A)Chloride
B)Phosphorus
C)Hydrogen
D)Sulfur

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
19
The atomic mass is calculated by:
A)adding the mass of the neutrons and protons.
B)subtracting the mass of electrons from the mass of the protons.
C)weighing individual atoms on microscales.
D)multiplying the mass of neutrons by the mass of the protons.
A)adding the mass of the neutrons and protons.
B)subtracting the mass of electrons from the mass of the protons.
C)weighing individual atoms on microscales.
D)multiplying the mass of neutrons by the mass of the protons.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an element?
A)Carbohydrates
B)Lipids
C)H₂O
D)Carbon
A)Carbohydrates
B)Lipids
C)H₂O
D)Carbon

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
21
What would happen if the active site of an enzyme had a different shape?
A)Nothing would change
B)The process would happen in reverse
C)The enzyme would die
D)The enzyme would not be functional
A)Nothing would change
B)The process would happen in reverse
C)The enzyme would die
D)The enzyme would not be functional

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
22
The negatively charged particle labeled C is called a(n)________. 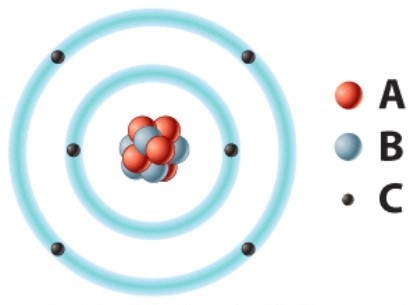
A)electron
B)neutron
C)proton
D)oxygen

A)electron
B)neutron
C)proton
D)oxygen

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
23
Which type of organic molecule is pictured here? 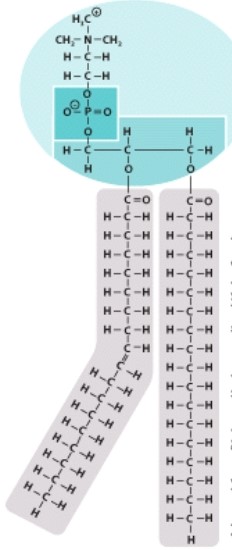
A)Carbohydrate
B)Lipid
C)Nucleic acid
D)Protein

A)Carbohydrate
B)Lipid
C)Nucleic acid
D)Protein

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following organic molecules can serve as a library of genetic information?
A)Carbohydrates
B)Proteins
C)Nucleic acids
D)Lipids
A)Carbohydrates
B)Proteins
C)Nucleic acids
D)Lipids

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following organic chemicals is the most abundant in your body?
A)Proteins
B)Lipids
C)Carbohydrates
D)Nucleic acids
A)Proteins
B)Lipids
C)Carbohydrates
D)Nucleic acids

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
26
Which type of lipid is a key component of the cell membrane?
A)Steroid
B)Phospholipid
C)Triglyceride
D)Fatty acid
A)Steroid
B)Phospholipid
C)Triglyceride
D)Fatty acid

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
27
An imbalance of the common ions found in the human body is life threatening.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
28
How are starch and cellulose different?
A)They are actually the same chemical known by two different names.
B)The branching pattern is different.
C)Animals make cellulose.
D)Plants use only starch.
A)They are actually the same chemical known by two different names.
B)The branching pattern is different.
C)Animals make cellulose.
D)Plants use only starch.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
29
The valence shell of an atom determines how many bonds it can form.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
30
Why is this organic molecule important in living organisms? 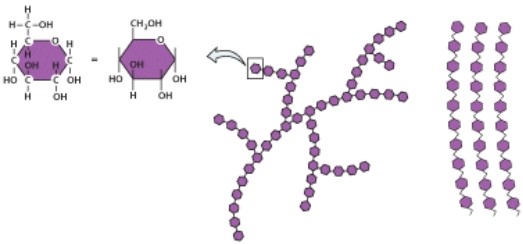
A)Structural component for most parts of a cell
B)Stores genetic information
C)Insulation
D)Biological energy

A)Structural component for most parts of a cell
B)Stores genetic information
C)Insulation
D)Biological energy

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
31
Which carbohydrate category does glucose belong to?
A)Polysaccharide
B)Disaccharide
C)Monosaccharide
D)Glycogen
A)Polysaccharide
B)Disaccharide
C)Monosaccharide
D)Glycogen

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
32
The arrow is pointing to a pH that is: 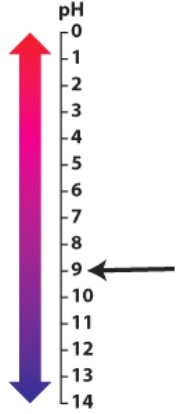
A)acidic.
B)neutral.
C)basic.
D)a buffer.

A)acidic.
B)neutral.
C)basic.
D)a buffer.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
33
What does the letter A represent in this diagram? 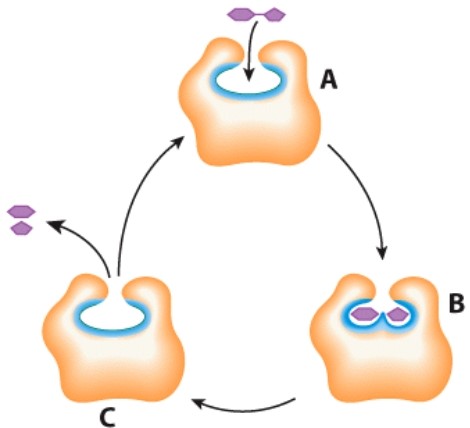
A)Enzyme
B)Energy
C)Products
D)Substrates

A)Enzyme
B)Energy
C)Products
D)Substrates

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
34
What does the letter C represent in this diagram? 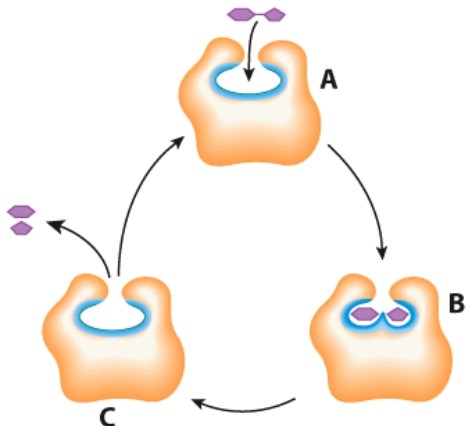
A)Enzyme
B)Product
C)Substrate
D)Acid

A)Enzyme
B)Product
C)Substrate
D)Acid

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
35
What kind of bond is indicated by the arrow in this diagram? 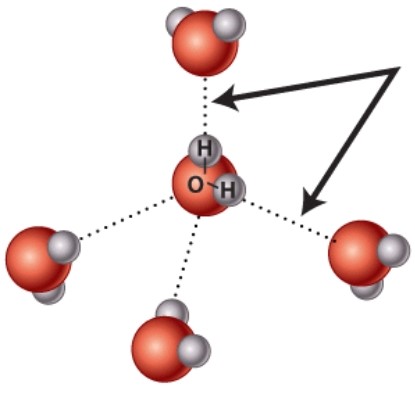
A)Covalent
B)Hydrogen
C)Ionic
D)Nonpolar

A)Covalent
B)Hydrogen
C)Ionic
D)Nonpolar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
36
What does the number 6 mean in this diagram? 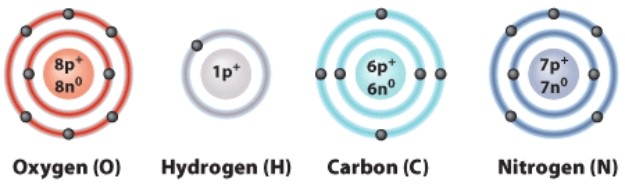
A)The weight of a carbon atom
B)The number of protons in a carbon atom
C)The number of electrons in a carbon ion
D)How many bonds carbon will form

A)The weight of a carbon atom
B)The number of protons in a carbon atom
C)The number of electrons in a carbon ion
D)How many bonds carbon will form

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
37
How many electrons are in the valence shell of the atom shown here? 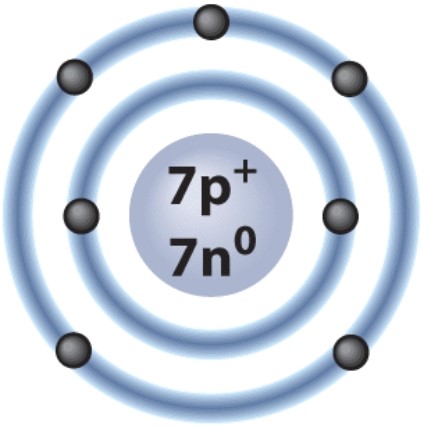
A)two
B)five
C)seven
D)four

A)two
B)five
C)seven
D)four

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
38
Using the numbers given in the diagram,how many neutrons does a carbon atom have? 
A)4
B)6
C)18
D)72

A)4
B)6
C)18
D)72

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
39
What kind of a bond is depicted in this diagram? 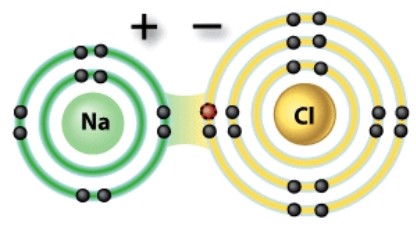
A)Covalent
B)Hydrogen
C)Ionic
D)Nonpolar

A)Covalent
B)Hydrogen
C)Ionic
D)Nonpolar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
40
The positively charged particle labeled A is called a(n)____. 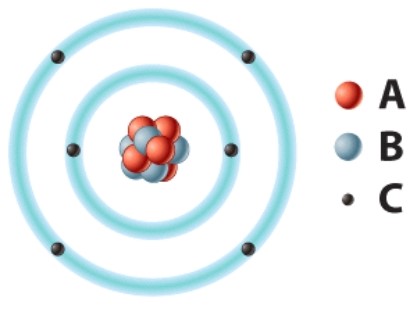
A)electron
B)neutron
C)proton
D)hydrogen

A)electron
B)neutron
C)proton
D)hydrogen

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
41
Water makes up about 33% of total body weight in humans.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
42
Ice floats because it is less dense than liquid water.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
43
Exothermic reactions like burning hydrogen fuel release energy.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
44
A positively charged ion is formed when an atom gains electrons.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
45
Hydrogen bonds hold the hydrogen and oxygen together in water molecules.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
46
Unsaturated fats are liquid at room temperature.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
47
Lye is a basic substance.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
48
Since carbon has 4 electrons in the outer energy level,it can form 4 covalent bonds.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
49
Hydrogen is not an atom because it does not have any neutrons.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck


