Deck 2: Earth Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 2: Earth Materials
1
What type of bond forms from the sharing of electrons between atoms?
A)covalent bond
B)metallic bond
C)ionic bond
D)Van der Waals bond
A)covalent bond
B)metallic bond
C)ionic bond
D)Van der Waals bond
A
2
The atomic number of an atom is
A)the number of protons and neutrons in the nucleus.
B)the number of protons in the nucleus.
C)the number of neutrons in the nucleus.
D)the number of electron energy levels.
A)the number of protons and neutrons in the nucleus.
B)the number of protons in the nucleus.
C)the number of neutrons in the nucleus.
D)the number of electron energy levels.
B
3
The property of a mineral to resist scratching is referred to as
A)streak.
B)density.
C)hardness.
D)tenacity.
A)streak.
B)density.
C)hardness.
D)tenacity.
C
4
Based on the definition of a mineral - being a naturally occurring solid,formed by inorganic processes,with a characteristic crystal structure and specific chemical composition,which materials will be classified as minerals?
A)water and ice
B)ice and steel
C)steel and coal
D)ice and quartz
A)water and ice
B)ice and steel
C)steel and coal
D)ice and quartz

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
The smallest particle that retains all of the chemical properties of an element is called a(n)
A)molecule.
B)isotope.
C)atom.
D)ion.
A)molecule.
B)isotope.
C)atom.
D)ion.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
The smallest chemical unit that has all the properties of a particular compound is called a(n)
A)molecule.
B)isotope.
C)atom.
D)ion.
A)molecule.
B)isotope.
C)atom.
D)ion.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
Atoms with the same atomic number but different mass numbers are called
A)molecules.
B)isotopes.
C)elements.
D)ions.
A)molecules.
B)isotopes.
C)elements.
D)ions.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
The mass number of an atom is:
A)the number of protons and neutrons in the nucleus.
B)the number of protons in the nucleus.
C)the number of neutrons in the nucleus.
D)the number of electron energy levels.
A)the number of protons and neutrons in the nucleus.
B)the number of protons in the nucleus.
C)the number of neutrons in the nucleus.
D)the number of electron energy levels.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
To which of the following groups do most minerals in Earth's crust belong?
A)oxides
B)halides
C)carbonates
D)silicates
A)oxides
B)halides
C)carbonates
D)silicates

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
Which element is the most abundant (by weight)in Earth's crust?
A)silicon
B)iron
C)calcium
D)oxygen
A)silicon
B)iron
C)calcium
D)oxygen

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
A hypothetical ion X has an electrical charge of -2.Which of the following statements best describes the relative number of electrons and protons in the atom?
A)The X ion has 2 less electrons than protons.
B)The X ion has 2 more electrons than protons.
C)The X ion has the same number of electrons as protons.
D)The X ion has 1 less electron than protons.
A)The X ion has 2 less electrons than protons.
B)The X ion has 2 more electrons than protons.
C)The X ion has the same number of electrons as protons.
D)The X ion has 1 less electron than protons.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
The property of a mineral that relates to how heavy it is for its size is referred to as:
A)streak.
B)density.
C)hardness.
D)tenacity
A)streak.
B)density.
C)hardness.
D)tenacity

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
In the illustration below,the part of the atom in which virtually all the mass is concentrated is referred to as the: 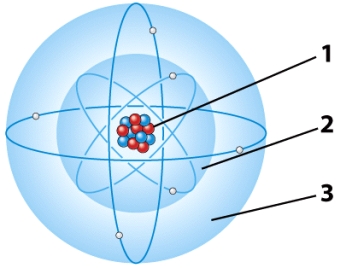
A)first electron energy level.
B)second electron energy level.
C)Both A and B are correct.
D)nucleus.

A)first electron energy level.
B)second electron energy level.
C)Both A and B are correct.
D)nucleus.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
The natural samples of corundum pictured below show variations of color from red (ruby)to blue (sapphire).The differences in color is best explained by: 
A)polymerization
B)crystal structure
C)the principle of atomic substitution
D)polymorphism

A)polymerization
B)crystal structure
C)the principle of atomic substitution
D)polymorphism

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
In the illustration below,the part(s)of the atom that has a negative charge is referred to as the: 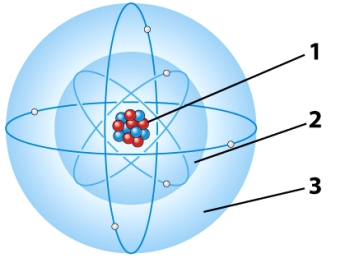
A)first electron energy level.
B)second electron energy level.
C)Both A and B are correct.
D)nucleus.

A)first electron energy level.
B)second electron energy level.
C)Both A and B are correct.
D)nucleus.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
What type of bond forms the strongest chemical bonds and compounds that tend to be strong with great hardness?
A)covalent bond
B)metallic bond
C)ionic bond
D)Van der Waals bond
A)covalent bond
B)metallic bond
C)ionic bond
D)Van der Waals bond

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
In the illustration below,the part of the atom that has a positive charge is referred to as the: 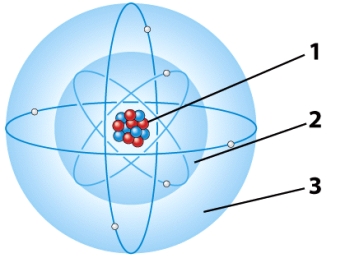
A)first electron energy level.
B)second electron energy level.
C)Both A and B are correct.
D)nucleus.

A)first electron energy level.
B)second electron energy level.
C)Both A and B are correct.
D)nucleus.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
One atom of a hypothetical element X has 27 protons while another has 29.This would make the two atoms of the element
A)molecules.
B)compounds.
C)ions.
D)isotopes.
A)molecules.
B)compounds.
C)ions.
D)isotopes.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
Why is steel not considered a mineral?
A)It is organic.
B)It is man-made.
C)It doesn't have a crystalline structure.
D)All of the above
A)It is organic.
B)It is man-made.
C)It doesn't have a crystalline structure.
D)All of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
What type of weak bond results from asymmetry in charge distribution?
A)covalent bond
B)metallic bond
C)ionic bond
D)Van der Waals bond
A)covalent bond
B)metallic bond
C)ionic bond
D)Van der Waals bond

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
The properties of compounds are not the same as the properties of their constituent elements.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the hardest silicate structure?
A)single tetrahedron
B)hexagonal ring
C)sheet
D)framework
A)single tetrahedron
B)hexagonal ring
C)sheet
D)framework

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
Which rock sample is held together by naturally forming cement? 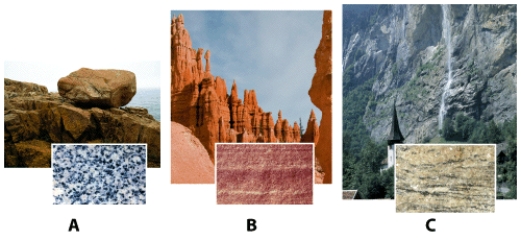
A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
A molecule is the smallest individual particle that retains the distinctive properties of an element.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
Minerals can be described in terms of two kinds of features: assemblage and texture.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
Which rock sample has been altered by heat and pressure so that a new mineral assemblage and rock fabric has developed? 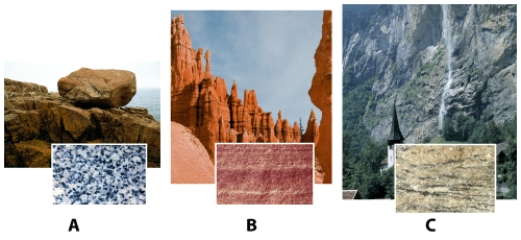
A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
What element bonds with a silicon atom to form the "silicate" tetrahedron?
A)oxygen
B)hydrogen
C)oxygen and hydrogen
D)carbon
A)oxygen
B)hydrogen
C)oxygen and hydrogen
D)carbon

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
Which sample is an example of sedimentary rock? 
A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
The color of a mineral is not necessarily useful in identification.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
In the photograph of the quartz crystal below,the angles between similar faces are constant because of the chemical composition of the mineral.
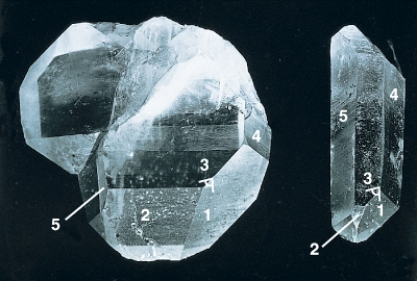


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
In ionic bonding,one atom may transfer electrons to another,creating ions with differing electrical charge.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
Which rock sample is formed from cooling and solidifying magma? 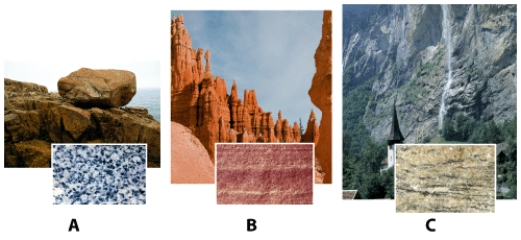
A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
Which sample is an example of an igneous rock? 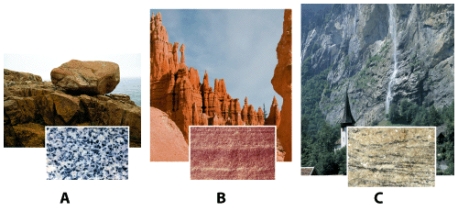
A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

A)Sample A.
B)Sample B.
C)Sample C.
D)None of the above answers are correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
Ionic bonds are the strongest chemical bonds,and elements and compounds with ionic bonds (such as diamond)tend to be strong and hard.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the weakest silicate structure?
A)single tetrahedron
B)hexagonal ring
C)sheet
D)framework
E)single chain
A)single tetrahedron
B)hexagonal ring
C)sheet
D)framework
E)single chain

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Silicon is the most abundant element in Earth's crust.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
The principle of atomic substitution is an exception to the rule that minerals have a specific chemical formula.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
What charge does the silicate ion contribute to form the silicate tetrahedron?
A)negative
B)no charge
C)positive
D)negative and positive
E)all of the above
A)negative
B)no charge
C)positive
D)negative and positive
E)all of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
A neutron is a positively charged particle with an atomic mass of 1,which resides in the nucleus of an atom.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
The two most common mineral families in Earth's crust are
A)the silicates and the oxides.
B)the silicates and the carbonates.
C)the carbonates and the oxides.
D)the phosphates and the oxides.
A)the silicates and the oxides.
B)the silicates and the carbonates.
C)the carbonates and the oxides.
D)the phosphates and the oxides.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
In ______,two elements are so similar in size and bonding properties that one can exchange for the other during crystallization.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
Sample (A,B,or C)_____ is a rock formed when mineral and rock particles are transported by water,wind,or ice and then deposited in a given location.Such rocks are classified as sedimentary rocks. 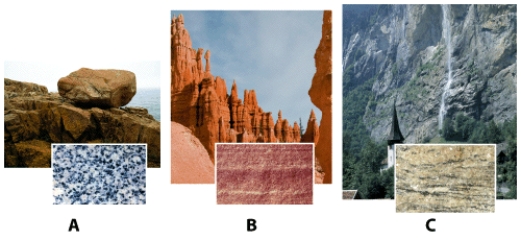


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
The property of ______ is a mineral's resistance to scratching.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
_____ and silicon are the two most common elements in Earth's crust.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
Based on their origins,rocks can be divided into three distinct families: _______,sedimentary,and metamorphic.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
The illustration below shows five different silicate structures.Of these,the structure labeled _____ exhibits the strongest structure for silicates.
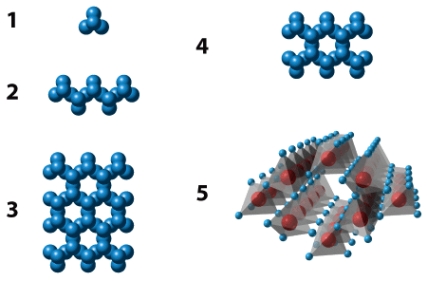


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
An atom that has an excess positive or negative electrical charge caused by the loss or addition of one or more electron is called a(n)______.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
The four types of bonding that are important in minerals are ______,covalent,metallic,and Van der Waals.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
The illustration below shows five different silicate structures.Of these,the structure labeled _____ is the most abundant in silicate minerals of Earth's crust.
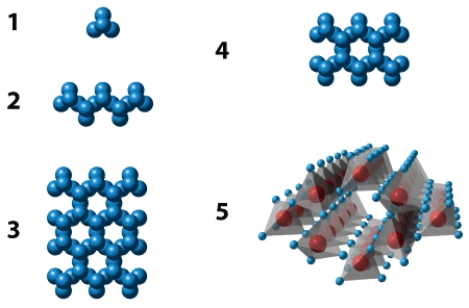


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
In the photograph of rock samples and outcrops,shown below,sample (A,B,or C)_____ is the rock held together by naturally occurring cement. 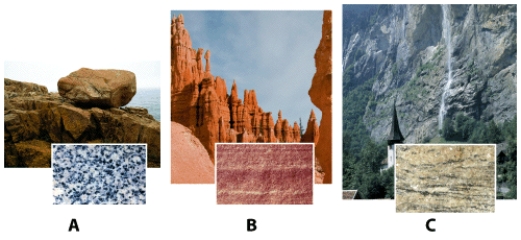


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
A(n)______ is the most fundamental substance into which matter can be separated by chemical means.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
Some minerals break along specific directions of weakness in their crystal structures.This property of a mineral to break in this predictable way is referred to as______.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Igneous rocks form from the cooling and solidification of magma.Sample (A,B,or C)_____ has formed by this processes. 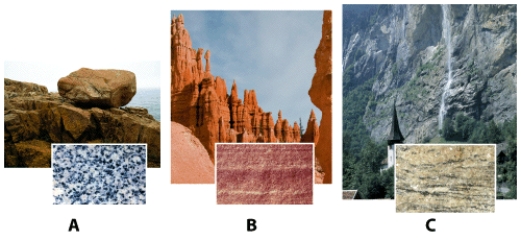


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
To be considered a mineral,a naturally occurring,inorganic solid must have a specific chemical composition and a characteristic ______ structure.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
The _____ of a mineral is the color of the mineral when powdered,which is usually accomplished in soft minerals by rubbing the sample against an unglazed porcelain plate.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
The mass number of an atom is the sum of the protons and ______.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
Sample (A,B,or C)_____ is of a rock whose original sedimentary or igneous form and mineral assemblage have been changed as a result of exposure to high temperature,high pressure or both.This type of rock is referred to as a metamorphic rock. 


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
The photo below shows two specimens of the mineral quartz.According to Steno's law the angle shown between faces 1 and 3 will be ______ in both samples.
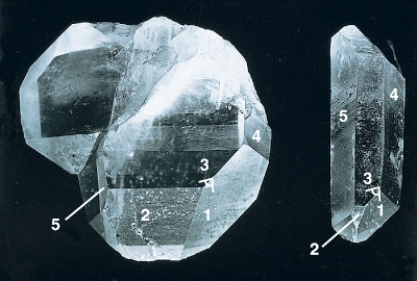


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
A(n)______ is the smallest chemical unit that retains all of the properties of a compound.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
The two most abundant mineral families of Earth's crust are the silicates and the _____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
What are the four requirements necessary to classify a solid material as a mineral?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
Discuss some of the ways in which the properties of minerals and rocks can affect our daily lives?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
Explain why it is not necessary to chemically analyze a common mineral in order to make an identification.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
What is the difference between ionic and covalent bonding? Which type of bonding creates compounds that tend to be relatively strong and hard?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
There are 8 protons in the nucleus of an oxygen atom.How many neutrons are there in oxygen 16,oxygen 17,and oxygen 18,respectively?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
What is the relationship between cleavage,crystal structure,and crystal faces? Speculate about how different types of bonding might influence each of these.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
The figure below illustrates bonding in two crystalline structures of carbon.Indicate which one is the structure of graphite and which diamond.Identify the type of bonding in each case and explain how the different bonding in the structures relates to the great difference in hardness between the two minerals.
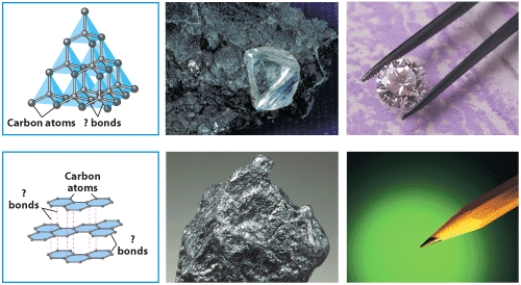


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
Two chemical elements make up 70 percent of Earth's crust by weight.What are the two elements,and what family of minerals do they form?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
With approximately 3,500 known minerals,why are there only about thirty common rock-forming minerals?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
Why is color an unreliable property to use when identifying a mineral?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
What's the difference between a rock and a mineral?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
What are the three families of rocks? How does each of these families differ?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
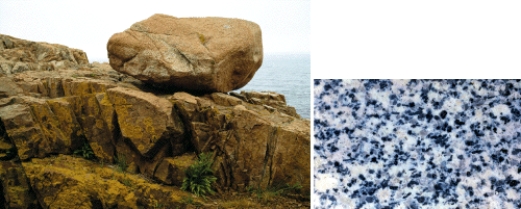
The photographs below show an outcrop and close up view of a particular kind of rock.What type of rock is it,and what holds this type of rock together? Also,explain how such a rock might form.


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
Crystals of the same type (i.e.quartz)all have a similar shape with flat crystals and specific angles between crystal faces.What controls this predictable geometry?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
What holds rocks together?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


