Deck 2: The Chemical Level of Organization
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 2: The Chemical Level of Organization
1
What region of an atom contains the protons and neutrons?
A)cloud
B)nucleus
C)element
D)ring
E)shell
A)cloud
B)nucleus
C)element
D)ring
E)shell
B
2
Describe the law of conservation of energy.
No Answer
3
Briefly describe the octet rule.
No Answer
4
The three types of subatomic particles that are important for understanding chemical reactions in the human body are
A)neutrons,quarks,and muons.
B)protons,neutrons,and electrons.
C)muons,positons,and neutrons.
D)electrons,quarks,and protons.
E)positons,protons,and neutrons.
A)neutrons,quarks,and muons.
B)protons,neutrons,and electrons.
C)muons,positons,and neutrons.
D)electrons,quarks,and protons.
E)positons,protons,and neutrons.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
What are the four major elements found in the chemicals that comprise the human body?
A)nitrogen,oxygen,calcium,sodium
B)hydrogen,carbon,phosphorus,calcium
C)carbon,hydrogen,oxygen and nitrogen
D)oxygen,nitrogen,potassium,calcium
E)potassium,phosphorus,sodium,hydrogen
A)nitrogen,oxygen,calcium,sodium
B)hydrogen,carbon,phosphorus,calcium
C)carbon,hydrogen,oxygen and nitrogen
D)oxygen,nitrogen,potassium,calcium
E)potassium,phosphorus,sodium,hydrogen

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
This is defined as the capacity to do work.
A)metabolism
B)electrolytes
C)chemical reaction
D)concentration
E)energy
A)metabolism
B)electrolytes
C)chemical reaction
D)concentration
E)energy

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
The chemical bonds formed between the atoms in a water molecule are called
A)nonpolar covalent bonds.
B)polar covalent bonds.
C)hydrogen bonds.
D)ionic bonds.
E)atomic bonds.
A)nonpolar covalent bonds.
B)polar covalent bonds.
C)hydrogen bonds.
D)ionic bonds.
E)atomic bonds.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
This type of chemical bond involves the sharing of valence electrons between two atoms.
A)covalent
B)ionic
C)hydrogen
D)atomic
E)electronic
A)covalent
B)ionic
C)hydrogen
D)atomic
E)electronic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
Describe a beneficial use of radiation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
This is the name given to a negatively charged atom.
A)superoxide
B)isotope
C)catalyst
D)anion
E)cation
A)superoxide
B)isotope
C)catalyst
D)anion
E)cation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
This refers to a weighted average of the atomic weights of all naturally occurring isotopes of an element.
A)mass number
B)atomic number
C)atomic mass
D)ionic mass
E)covalent mass
A)mass number
B)atomic number
C)atomic mass
D)ionic mass
E)covalent mass

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following subatomic particles are shared by two atoms to form covalent bonds??1.neutron?2.electron?3.proton
A)1 only
B)2 only
C)3 only
D)2 & 3 only
E)1,2 & 3
A)1 only
B)2 only
C)3 only
D)2 & 3 only
E)1,2 & 3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
A chemical that can conduct electrical current when dissolved in water is called a(n)
A)isotope.
B)isomer.
C)compound.
D)electrolyte
E)valence molecule.
A)isotope.
B)isomer.
C)compound.
D)electrolyte
E)valence molecule.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following subatomic particles has a neutral charge?
A)neutron
B)electron
C)proton
D)Both neutron and electron.
E)All of these choices.
A)neutron
B)electron
C)proton
D)Both neutron and electron.
E)All of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
The nucleus of unstable _____ of an element will decay leading to emission of radiation.
A)compounds
B)cations
C)anions
D)isotopes
E)molecules
A)compounds
B)cations
C)anions
D)isotopes
E)molecules

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
This relatively weak type of bond helps stabilize the three dimensional structure of large molecules like proteins and DNA?
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic
E)atomic
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic
E)atomic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The number of protons in an atom is represented by an element's
A)mass number.
B)atomic number.
C)atomic mass.
D)valence number.
E)None of these choices.
A)mass number.
B)atomic number.
C)atomic mass.
D)valence number.
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
A chemical reaction involves interactions between the _______ of two different atoms.
A)neutrons
B)protons
C)isotopes
D)valence electrons
E)ions
A)neutrons
B)protons
C)isotopes
D)valence electrons
E)ions

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
This type of chemical reaction will absorb more energy than it releases.
A)exergonic
B)endergonic
C)potential
D)kinetic
E)activation
A)exergonic
B)endergonic
C)potential
D)kinetic
E)activation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Describe a hydrogen bond.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
This is the most abundant and most important inorganic compound in the body.
A)water
B)oxygen gas
C)carbon dioxide
D)glucose
E)DNA
A)water
B)oxygen gas
C)carbon dioxide
D)glucose
E)DNA

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
This type of fatty acid contains more than one double bond in its hydrocarbon chain.
A)saturated
B)monounsaturated
C)polyunsaturated
D)volatile
E)short chain
A)saturated
B)monounsaturated
C)polyunsaturated
D)volatile
E)short chain

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a monosaccharide that is used by cells to produce energy?
A)glucose
B)sucrose
C)lactose
D)glycogen
E)maltose
A)glucose
B)sucrose
C)lactose
D)glycogen
E)maltose

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Describe the functions of water in the body.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is NOT true about phospholipids?
A)They contain a glycerol backbone.
B)The head group is polar.
C)The molecule is an important part of cell membranes.
D)The tail groups are nonpolar.
E)They are a major form of energy storage.
A)They contain a glycerol backbone.
B)The head group is polar.
C)The molecule is an important part of cell membranes.
D)The tail groups are nonpolar.
E)They are a major form of energy storage.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a proton donor?
A)acid
B)base
C)salt
D)organic compound
E)colloid
A)acid
B)base
C)salt
D)organic compound
E)colloid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Prostaglandins and leukotrienes are?1 synthesized from cholesterol.?2 lipids.?3 eicosanoids.
A)1 only
B)2 only
C)3 only
D)Both 2 and 3
E)1,2 and 3
A)1 only
B)2 only
C)3 only
D)Both 2 and 3
E)1,2 and 3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Specific arrangements of atoms within an organic molecule that confer characteristic chemical properties upon that molecule are called
A)hydrocarbon chains.
B)polymers.
C)carbon skeleton.
D)functional groups.
E)isomers.
A)hydrocarbon chains.
B)polymers.
C)carbon skeleton.
D)functional groups.
E)isomers.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
This type of chemical reaction combines reactants to produce larger products.
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
This lipid is used by the body as a precursor for the production of steroid hormones.
A)arachidonic acid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein
A)arachidonic acid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
An enzyme acts to
A)raise the activation energy needed to start the reaction.
B)lower the activation energy needed to start the reaction.
C)convert the activation energy into potential energy.
D)convert the activation energy into kinetic energy.
E)stop a chemical reaction.
A)raise the activation energy needed to start the reaction.
B)lower the activation energy needed to start the reaction.
C)convert the activation energy into potential energy.
D)convert the activation energy into kinetic energy.
E)stop a chemical reaction.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
A chemical compound that helps control the pH of a solution by adding or removing hydrogen ions is a(n)
A)electrolyte.
B)salt.
C)cation.
D)colloid.
E)buffer.
A)electrolyte.
B)salt.
C)cation.
D)colloid.
E)buffer.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
This type of lipid is the body's primary long-term energy storage molecule.
A)steroid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein
A)steroid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Glucose and fructose both have the chemical formula (C₆H₁₂O₆)so they are considered
A)isotopes.
B)polymers.
C)monomers.
D)isomers.
E)isogenic.
A)isotopes.
B)polymers.
C)monomers.
D)isomers.
E)isogenic.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
In the body fluid compartments found in the human body,the solvent is
A)glucose.
B)lipids.
C)carbon dioxide.
D)water.
E)electrolyte.
A)glucose.
B)lipids.
C)carbon dioxide.
D)water.
E)electrolyte.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
List three factors that increase the rate of chemical reactions.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
A solute that readily dissolves in water is
A)hydrophobic.
B)hydrostatic.
C)lipophilic.
D)hydrophilic.
E)hydrozone.
A)hydrophobic.
B)hydrostatic.
C)lipophilic.
D)hydrophilic.
E)hydrozone.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
This type of chemical reaction breaks larger reactants into smaller products.
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is a polysaccharide that serves as a storage form of energy in muscle and liver cells?
A)cellulose
B)ribose
C)lipids
D)glucose
E)glycogen
A)cellulose
B)ribose
C)lipids
D)glucose
E)glycogen

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
A solution with a pH value less than 7 is
A)basic.
B)neutral.
C)acidic.
D)alkaline.
E)concentrated.
A)basic.
B)neutral.
C)acidic.
D)alkaline.
E)concentrated.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
In the diagram,which labeled structure is a pyrimidine base? 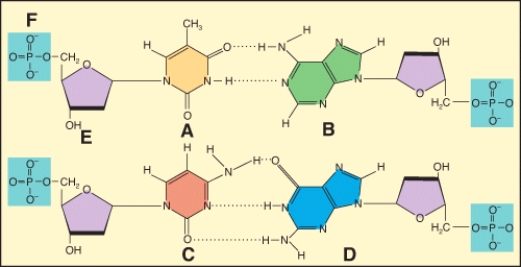
A)A
B)B
C)E
D)F
E)None of these choices.

A)A
B)B
C)E
D)F
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
In the diagram,which particles are found in the atom's nucleus? 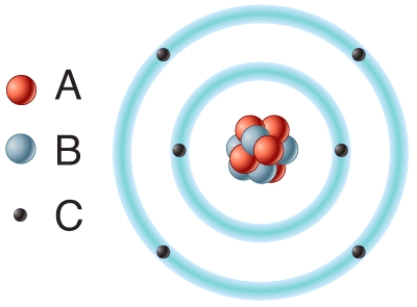
A)A
B)B
C)C
D)Both A and B.
E)None of these choices.

A)A
B)B
C)C
D)Both A and B.
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
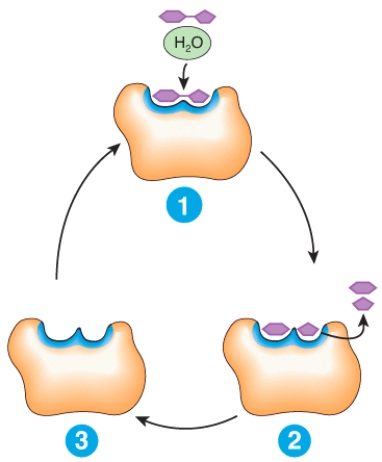
Describe what is happening at places 1,2 and 3 in the diagram.


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
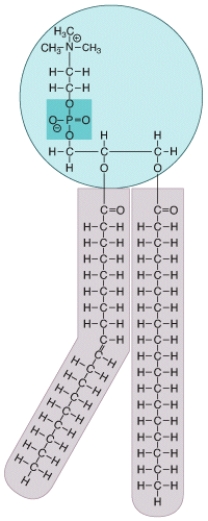
What type of molecule is shown in the diagram? Where in a eukaryotic cell would this type of molecule be commonly found? What special chemical properties does this molecule possess that allows it to accomplish its functions?


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
In the diagram,removal of one or more of this type of subatomic particle would result in the formation of a cation? 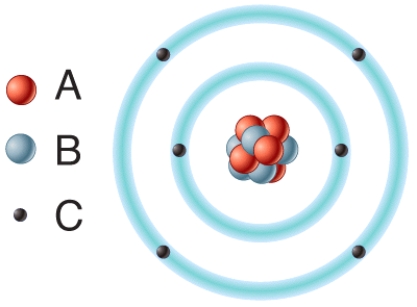
A)A
B)B
C)C
D)All of these choices.
E)None of these choices.

A)A
B)B
C)C
D)All of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the major function of DNA?
A)catalyzes metabolic reactions
B)storage of energy
C)transfer information for protein synthesis
D)long-term storage of information for protein synthesis
E)transport of electrolytes
A)catalyzes metabolic reactions
B)storage of energy
C)transfer information for protein synthesis
D)long-term storage of information for protein synthesis
E)transport of electrolytes

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
In the diagram,which labeled structure is a purine base? 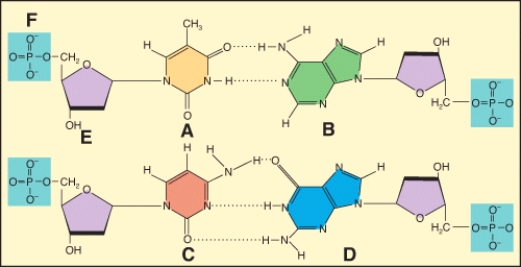
A)A
B)B
C)E
D)Both A and B.
E)All of these choices

A)A
B)B
C)E
D)Both A and B.
E)All of these choices

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is NOT a property of enzymes?
A)Enzymes are catalytic proteins.
B)Enzymes are highly specific.
C)Enzymes are efficient.
D)Enzymes are subject to a variety of cellular controls.
E)Enzymes are irreversibly changed by the reactions that they catalyze.
A)Enzymes are catalytic proteins.
B)Enzymes are highly specific.
C)Enzymes are efficient.
D)Enzymes are subject to a variety of cellular controls.
E)Enzymes are irreversibly changed by the reactions that they catalyze.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
In the diagram which particles are negatively charged? 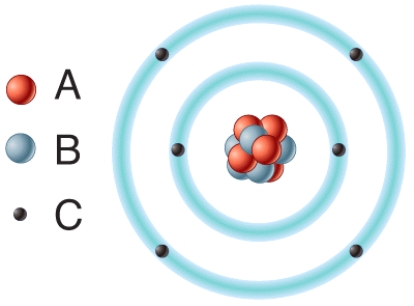
A)A
B)B
C)C
D)All of these choices.
E)None of these choices.

A)A
B)B
C)C
D)All of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the labeled structures are found in DNA but not RNA????1 A?2 B?3 C?4 E 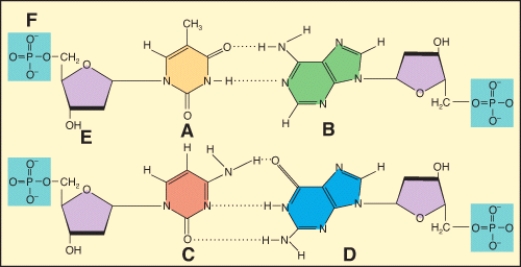
A)1 only
B)2 only
C)3 only
D)4 only
E)1 and 4

A)1 only
B)2 only
C)3 only
D)4 only
E)1 and 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
The primary structure of a protein consists of
A)alpha helices.
B)beta-pleated sheets.
C)three dimensional folded conformation.
D)a sequence of amino acids linked by peptide bonds.
E)the overall folded conformation of the protein's subunits.
A)alpha helices.
B)beta-pleated sheets.
C)three dimensional folded conformation.
D)a sequence of amino acids linked by peptide bonds.
E)the overall folded conformation of the protein's subunits.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is a purine base?
A)cytosine
B)guanine
C)thymine
D)uracil
E)None of these choices.
A)cytosine
B)guanine
C)thymine
D)uracil
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is a common function of RNA?
A)produce electrical impulses
B)storage of energy
C)transfer information for protein synthesis
D)long-term storage of information for protein synthesis
E)transport of fluids
A)produce electrical impulses
B)storage of energy
C)transfer information for protein synthesis
D)long-term storage of information for protein synthesis
E)transport of fluids

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Describe what happens to a protein's structure and function when it is denatured.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
Describe the structural characteristics of an amino acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Which monomer is used to build RNA and DNA?
A)fatty acid
B)amino acid
C)monosaccharide
D)glycerol
E)nucleotide
A)fatty acid
B)amino acid
C)monosaccharide
D)glycerol
E)nucleotide

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
List the six major functions of proteins.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
In the diagram,which labeled structure is deoxyribose? 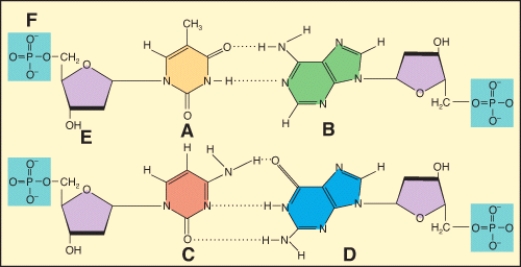
A)B
B)E
C)F
D)None of these choices.
E)The entire structure is considered a deoxyribose molecule.

A)B
B)E
C)F
D)None of these choices.
E)The entire structure is considered a deoxyribose molecule.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
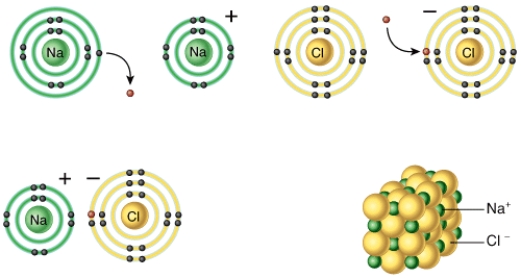
Describe the basic chemical principle that is shown in this diagram?


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following describes the major function of ATP in cells?
A)building block for the synthesis of proteins.
B)transfers energy for cell functions
C)transfers information for protein synthesis
D)stores information for protein synthesis
E)transports fluids
A)building block for the synthesis of proteins.
B)transfers energy for cell functions
C)transfers information for protein synthesis
D)stores information for protein synthesis
E)transports fluids

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is NOT an antioxidant?
A)selenium
B)zinc
C)superoxide
D)vitamin C
E)beta-carotene
A)selenium
B)zinc
C)superoxide
D)vitamin C
E)beta-carotene

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following chemical elements present in the body is considered a lesser element?
A)nitrogen
B)oxygen
C)calcium
D)iodine
E)chromium
A)nitrogen
B)oxygen
C)calcium
D)iodine
E)chromium

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following chemical elements present in the body is NOT a trace element?
A)chlorine
B)zinc
C)selenium
D)iodine
E)boron
A)chlorine
B)zinc
C)selenium
D)iodine
E)boron

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following carbohydrates is a disaccharide?
A)ribose
B)lactose
C)galactose
D)glycogen
E)cellulose
A)ribose
B)lactose
C)galactose
D)glycogen
E)cellulose

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following chemical elements present in the body is considered a lesser element?
A)phosphorus
B)oxygen
C)nitrogen
D)aluminum
E)copper
A)phosphorus
B)oxygen
C)nitrogen
D)aluminum
E)copper

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following chemical elements present in the body is NOT considered a lesser element?
A)sulfur
B)oxygen
C)magnesium
D)potassium
E)calcium
A)sulfur
B)oxygen
C)magnesium
D)potassium
E)calcium

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following describes the major significance of the element chlorine in the human body?
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)forms backbone of all organic molecules
D)required for bone and tooth structure
E)ionized form is most plentiful cation in extracellular fluid
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)forms backbone of all organic molecules
D)required for bone and tooth structure
E)ionized form is most plentiful cation in extracellular fluid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
In the diagram,what would happen to the concentration of C if the concentration of A increases? 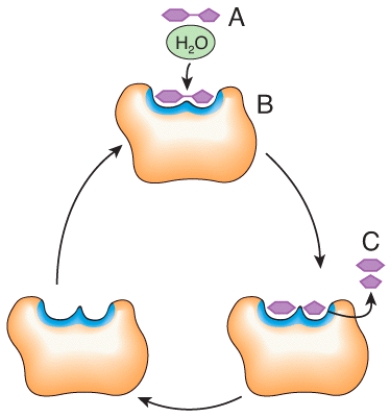
A)increases
B)decreases
C)no change

A)increases
B)decreases
C)no change

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following describes the major significance of the element nitrogen in the human body?
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)ionized form is needed for action of many enzymes
D)is a component of all proteins and nucleic acids
E)ionized form is most plentiful cation in extracellular fluid
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)ionized form is needed for action of many enzymes
D)is a component of all proteins and nucleic acids
E)ionized form is most plentiful cation in extracellular fluid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following substances has a pH closest to 7.0?
A)lye
B)vaginal fluid
C)semen
D)gastric juice
E)milk of magnesia
A)lye
B)vaginal fluid
C)semen
D)gastric juice
E)milk of magnesia

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following can lower the amount of free radicals in the body?
A)x-rays
B)ultraviolet radiation
C)oxygen
D)carbon tetrachloride
E)antioxidants
A)x-rays
B)ultraviolet radiation
C)oxygen
D)carbon tetrachloride
E)antioxidants

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following chemical elements present in the body is considered a trace element?
A)chlorine
B)oxygen
C)iron
D)iodine
E)nitrogen
A)chlorine
B)oxygen
C)iron
D)iodine
E)nitrogen

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
In the diagram,what pH value represents an acidic solution? 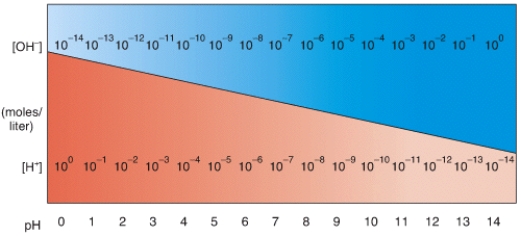
A)12
B)10
C)8
D)6
E)None of these choices.

A)12
B)10
C)8
D)6
E)None of these choices.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following illustrates the use of a radioisotope as a tracer?
A)monitor blood flow through the heart
B)shrink a tumor by killing tumor cells with emitted radiation
C)destroy overactive thyroid gland
D)treat prostate cancer
E)treat advanced cervical cancer
A)monitor blood flow through the heart
B)shrink a tumor by killing tumor cells with emitted radiation
C)destroy overactive thyroid gland
D)treat prostate cancer
E)treat advanced cervical cancer

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following describes the major significance of the element carbon in the human body?
A)ionized form makes body fluids acidic
B)constituent of water
C)forms backbone of all organic molecules
D)required to harden the structure of bones and teeth
E)ionized form is the part of hemoglobin that carries oxygen
A)ionized form makes body fluids acidic
B)constituent of water
C)forms backbone of all organic molecules
D)required to harden the structure of bones and teeth
E)ionized form is the part of hemoglobin that carries oxygen

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following describes the major significance of the element magnesium in the human body?
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)ionized form is needed for action of many enzymes
D)is a component of all proteins and nucleic acids
E)ionized form is most plentiful cation in extracellular fluid
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)ionized form is needed for action of many enzymes
D)is a component of all proteins and nucleic acids
E)ionized form is most plentiful cation in extracellular fluid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following carbohydrates is a polysaccharide?
A)ribose
B)lactose
C)glycogen
D)maltose
E)galactose
A)ribose
B)lactose
C)glycogen
D)maltose
E)galactose

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following describes the major significance of the element iron in the human body?
A)ionized form makes body fluids acidic
B)used to generate ATP
C)forms backbone of all organic molecules
D)required for bone and tooth structure
E)ionized forms are part of hemoglobin
A)ionized form makes body fluids acidic
B)used to generate ATP
C)forms backbone of all organic molecules
D)required for bone and tooth structure
E)ionized forms are part of hemoglobin

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following substances has a pH closest to 7.0?
A)lye
B)vaginal fluid
C)gastric juice
D)cerebrospinal fluid
E)milk of magnesia
A)lye
B)vaginal fluid
C)gastric juice
D)cerebrospinal fluid
E)milk of magnesia

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
What is the difference between atomic mass,mass number and atomic number?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


