Deck 7: Mass Stoichiometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 7: Mass Stoichiometry
1
What is the formula mass,to the nearest whole number,for potassium nitrite,KNO2?
A) 39 amu
B) 69 amu
C) 85 amu
D) 153 amu
A) 39 amu
B) 69 amu
C) 85 amu
D) 153 amu
85 amu
2
How many moles of KF are present in 15.2 grams of KF?
A) 0.262
B) 3.82
C) 6.02
D) 882
A) 0.262
B) 3.82
C) 6.02
D) 882
0.262
3
How many moles of CO2 are present in 56.0 grams of CO2?
A) 0.786
B) 2.00
C) 0.560
D) 1.27
A) 0.786
B) 2.00
C) 0.560
D) 1.27
1.27
4
How many atoms are present in a 0.900-mole sample of gold?
A) 1.81 × 1023
B) 1.50 × 1024
C) 5.42 × 1023
D) 6.02 × 1023
A) 1.81 × 1023
B) 1.50 × 1024
C) 5.42 × 1023
D) 6.02 × 1023

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
Lithium carbonate,Li2CO3,is commonly used to treat bipolar disorder.What is the percent by mass of carbon in this compound (to the nearest whole percent)?
A) 8%
B) 16%
C) 58%
D) 75%
A) 8%
B) 16%
C) 58%
D) 75%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
A skunk's spray contains the foul-smelling compound butanethiol,C4H8S.What is the percent by mass of sulfur in this compound (to the nearest whole percent)?
A) 8%
B) 36%
C) 55%
D) 75%
A) 8%
B) 36%
C) 55%
D) 75%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
What is the formula mass,to the nearest whole number,for sodium nitrite,NaNO2?
A) 23 amu
B) 53 amu
C) 69 amu
D) 83 amu
A) 23 amu
B) 53 amu
C) 69 amu
D) 83 amu

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
How many molecules of carbon monoxide,CO,comprise 2.34 moles?
A) 1.41 × 1024
B) 2.57 × 1023
C) 1.69 × 1025
D) 2.15 × 1022
A) 1.41 × 1024
B) 2.57 × 1023
C) 1.69 × 1025
D) 2.15 × 1022

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
How many atoms are present in a 0.300-mole sample of gold?
A) 4.98 × 1025
B) 1.27 × 1015
C) 1.81 × 1023
D) 6.02 × 1023
A) 4.98 × 1025
B) 1.27 × 1015
C) 1.81 × 1023
D) 6.02 × 1023

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
How many molecules of carbon dioxide,CO2,comprise 1.22 moles?
A) 7.34 × 1023
B) 4.93 × 1023
C) 2.65 × 1025
D) 1.37 × 1022
A) 7.34 × 1023
B) 4.93 × 1023
C) 2.65 × 1025
D) 1.37 × 1022

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
A skunk's spray contains the foul-smelling compound butanethiol,C4H8S.What is the percent by mass of carbon in this compound (to the nearest whole percent)?
A) 8%
B) 31%
C) 55%
D) 75%
A) 8%
B) 31%
C) 55%
D) 75%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
How many atoms are present in a 0.600-mole sample of gold?
A) 3.61 × 1023
B) 1.18 × 1015
C) 9.97 × 1025
D) 6.02 × 1023
A) 3.61 × 1023
B) 1.18 × 1015
C) 9.97 × 1025
D) 6.02 × 1023

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
What is the mass of 1.6 × 1021 argon atoms?
A) 0.11 g
B) 0.0027 g
C) 39.4 g
D) 5.11 × 1022 g
A) 0.11 g
B) 0.0027 g
C) 39.4 g
D) 5.11 × 1022 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
How many moles of CO2 are present in 88.0 grams of CO2?
A) 0.636
B) 1.42
C) 0.88
D) 2.00
A) 0.636
B) 1.42
C) 0.88
D) 2.00

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
What is the formula mass,to the nearest whole number,for lithium nitrite,LiNO2?
A) 7 amu
B) 44 amu
C) 37 amu
D) 53 amu
A) 7 amu
B) 44 amu
C) 37 amu
D) 53 amu

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
What is the mass of 1.5 × 1021 silicon atoms?
A) 0.0025 g
B) 4.21 × 1022 g
C) 0.070 g
D) 9.52 × 1024 g
A) 0.0025 g
B) 4.21 × 1022 g
C) 0.070 g
D) 9.52 × 1024 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
What is the mass of 3.00 moles of CS2?
A) 25.4 g
B) 229 g
C) 0.0394 g
D) 1.81 × 1024 g
A) 25.4 g
B) 229 g
C) 0.0394 g
D) 1.81 × 1024 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
What is the mass of 3.20 moles of KF?
A) 18.2 g
B) 0.055 g
C) 186 g
D) 1.93 × 1024 g
A) 18.2 g
B) 0.055 g
C) 186 g
D) 1.93 × 1024 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
How many molecules of water,H2O,comprise 2.07 moles?
A) 1.25 × 1024
B) 2.91 × 1023
C) 1.08 × 1025
D) 3.34 × 1022
A) 1.25 × 1024
B) 2.91 × 1023
C) 1.08 × 1025
D) 3.34 × 1022

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
What is the mass of 2.00 moles of CS2?
A) 38.1 g
B) 152 g
C) 0.0262 g
D) 1.20 × 1024 g
A) 38.1 g
B) 152 g
C) 0.0262 g
D) 1.20 × 1024 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Based on the following balanced equation,how many moles of O2 will be needed to react completely with of 4 moles of CH4? CH4 (g)+ 2 O2 (g) CO2 (g)+ 2 H2O (g)
A) 2
B) 4
C) 8
D) 12
A) 2
B) 4
C) 8
D) 12

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
In this reaction,the symbol (aq)after BaCl2 means that it is: BaCl2 (aq)+ Na2CO3 (aq) BaCO3 (s)+ 2 NaCl (aq)
A) the excess reagent.
B) an ionic compound.
C) a reactant.
D) dissolved in water.
A) the excess reagent.
B) an ionic compound.
C) a reactant.
D) dissolved in water.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
In this reaction,BaCO3 is the: BaCl2 (aq)+ Na2CO3 (aq) BaCO3 (s)+ 2 NaCl (aq)
A) excess reagent.
B) reactant.
C) dissociated compound.
D) precipitate.
A) excess reagent.
B) reactant.
C) dissociated compound.
D) precipitate.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the following reaction: Fe + Br2 FeBr2
If 44.2 grams of iron react with an excess of bromine gas,what mass of FeBr2 can form?
(FeBr2 = 215.65 g/mol)
A) 171 g
B) 3.67 × 10-3 g
C) 11.4 g
D) 272 g
If 44.2 grams of iron react with an excess of bromine gas,what mass of FeBr2 can form?
(FeBr2 = 215.65 g/mol)
A) 171 g
B) 3.67 × 10-3 g
C) 11.4 g
D) 272 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Based on the following balanced equation,how many moles of H2O can form from the reaction of 6 moles of CH4? CH4 (g)+ 2 O2 (g) CO2 (g)+ 2 H2O (g)
A) 6
B) 3
C) 12
D) 18
A) 6
B) 3
C) 12
D) 18

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
In this reaction,the limiting reagent is: 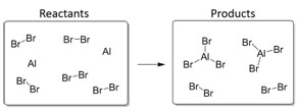
A) Br2.
B) AlBr3.
C) Al.
D) H2O.

A) Br2.
B) AlBr3.
C) Al.
D) H2O.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Based on the following balanced equation,how many moles of CO2 will be produced along with 6 moles of water? CH4 (g)+ 2 O2 (g) CO2 (g)+ 2 H2O (g)
A) 2
B) 3
C) 4
D) 8
A) 2
B) 3
C) 4
D) 8

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
In this reaction,the symbol (aq)after ZnBr2 means that it is: ZnBr2 (aq)+ K2CO3 (aq) 2 KBr (aq)+ ZnCO3 (s)
A) the excess reagent.
B) an ionic compound.
C) a reactant.
D) dissolved in water.
A) the excess reagent.
B) an ionic compound.
C) a reactant.
D) dissolved in water.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the following reaction: 4 Fe + 3 O2 2 Fe2O3
If 44.2 grams of iron react with an excess of oxygen gas,what mass of Fe2O3 can form?
(Fe2O3 = 159.70 g/mol)
A) 63.2 g
B) 253 g
C) 15.5 g
D) 126 g
If 44.2 grams of iron react with an excess of oxygen gas,what mass of Fe2O3 can form?
(Fe2O3 = 159.70 g/mol)
A) 63.2 g
B) 253 g
C) 15.5 g
D) 126 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
Based on the following balanced equation,how many moles of SF6 can form from the combination of 6 moles of S and 15 moles of F2? S + 3 F2 SF6
A) 5
B) 6
C) 15
D) 20
A) 5
B) 6
C) 15
D) 20

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the following reaction: Fe + Br2 FeBr2
If 53.2 grams of iron react with an excess of bromine gas,what mass of FeBr2 can form?
(FeBr2 = 215.65 g/mol)
A) 226 g
B) 13.8 g
C) 205 g
D) 4.41 × 10-3 g
If 53.2 grams of iron react with an excess of bromine gas,what mass of FeBr2 can form?
(FeBr2 = 215.65 g/mol)
A) 226 g
B) 13.8 g
C) 205 g
D) 4.41 × 10-3 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
How many molecules of water,H2O,comprise 3.64 grams?
A) 1.22 × 1023
B) 3.95 × 1025
C) 2.98 × 1024
D) 8.22 × 1024
A) 1.22 × 1023
B) 3.95 × 1025
C) 2.98 × 1024
D) 8.22 × 1024

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the following reaction: Fe + Br2 FeBr2
If 38.6 grams of iron react with an excess of bromine gas,what mass of FeBr2 can form?
(FeBr2 = 215.65 g/mol)
A) 149 g
B) 3.20 × 10-3 g
C) 10.0 g
D) 312 g
If 38.6 grams of iron react with an excess of bromine gas,what mass of FeBr2 can form?
(FeBr2 = 215.65 g/mol)
A) 149 g
B) 3.20 × 10-3 g
C) 10.0 g
D) 312 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
How many molecules of carbon dioxide,CO2,comprise 2.55 grams?
A) 3.49 × 1022
B) 6.76 × 1025
C) 1.04 × 1025
D) 2.86 × 1023
A) 3.49 × 1022
B) 6.76 × 1025
C) 1.04 × 1025
D) 2.86 × 1023

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Based on the following balanced equation,how many moles of SF6 can form from the combination of 5 moles of S and 18 moles of F2? S + 3 F2 SF6
A) 6
B) 5
C) 18
D) 23
A) 6
B) 5
C) 18
D) 23

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
In this reaction,the excess reagent is: 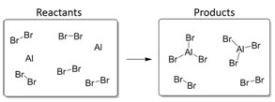
A) Br2.
B) AlBr3.
C) Al.
D) H2O.

A) Br2.
B) AlBr3.
C) Al.
D) H2O.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
How many molecules of carbon monoxide,CO,comprise 4.82 grams?
A) 1.04 × 1023
B) 8.13 × 1025
C) 3.50 × 1024
D) 2.24 × 1022
A) 1.04 × 1023
B) 8.13 × 1025
C) 3.50 × 1024
D) 2.24 × 1022

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
In this reaction,ZnCO3 is the: ZnBr2 (aq)+ K2CO3 (aq) 2 KBr (aq)+ ZnCO3 (s)
A) precipitate.
B) reactant.
C) dissociated compound.
D) excess reagent.
A) precipitate.
B) reactant.
C) dissociated compound.
D) excess reagent.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Based on the following balanced equation,how many moles of SF6 can form from the combination of 11 moles of S and 18 moles of F2? S + 3 F2 SF6
A) 6
B) 11
C) 18
D) 29
A) 6
B) 11
C) 18
D) 29

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
What is the mass of 1.6 × 1021 neon atoms?
A) 0.054 g
B) 0.0027 g
C) 19 g
D) 3.2 × 1022 g
A) 0.054 g
B) 0.0027 g
C) 19 g
D) 3.2 × 1022 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
A chemist carries out this reaction in the laboratory using 4.31 grams of zinc and an excess of sulfur: Zn + S ZnS
From the balanced equation,she calculates that she should obtain 6.41 grams of zinc sulfide.However,she isolates only 5.01 grams of product.What is her percent yield for this reaction?
A) 67.2%
B) 86.0%
C) 78.2%
D) 128%
From the balanced equation,she calculates that she should obtain 6.41 grams of zinc sulfide.However,she isolates only 5.01 grams of product.What is her percent yield for this reaction?
A) 67.2%
B) 86.0%
C) 78.2%
D) 128%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following reaction: 4 Fe + 3 O2 2 Fe2O3
If 44.2 grams of oxygen react with an excess of iron,what mass of Fe2O3 can form?
(Fe2O3 = 159.70 g/mol)
A) 147 g
B) 331 g
C) 294 g
D) 221 g
If 44.2 grams of oxygen react with an excess of iron,what mass of Fe2O3 can form?
(Fe2O3 = 159.70 g/mol)
A) 147 g
B) 331 g
C) 294 g
D) 221 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
A chemist carries out this reaction in the laboratory using 4.31 grams of zinc and an excess of sulfur: Zn + S ZnS
From the balanced equation,she calculates that she should obtain 6.41 grams of zinc sulfide.However,she isolates only 5.85 grams of product.What is her percent yield for this reaction?
A) 2.1%
B) 67.2%
C) 109.6%
D) 91.3%
From the balanced equation,she calculates that she should obtain 6.41 grams of zinc sulfide.However,she isolates only 5.85 grams of product.What is her percent yield for this reaction?
A) 2.1%
B) 67.2%
C) 109.6%
D) 91.3%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following reaction: 4 Fe + 3 O2 2 Fe2O3
How many grams of oxygen gas are needed to react completely with 44.2 grams of iron?
A) 19.0 g
B) 101 g
C) 12.7 g
D) 25.3 g
How many grams of oxygen gas are needed to react completely with 44.2 grams of iron?
A) 19.0 g
B) 101 g
C) 12.7 g
D) 25.3 g

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


