Deck 16: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/137
Play
Full screen (f)
Deck 16: Acids and Bases
1
According to the Arrhenius theory,a neutralization reaction involves the combination of an acid with a base to make only water.
False
2
The first ionization step is approximately 100% for H2SO3.
False
3
The term pH = -ln [H+].
False
4
According to the Arrhenius theory,a neutralization reaction involves:
A)the combination of hydrogen ions and hydroxide ions to form water
B)the dissociation of a strong acid to hydrogen ions and an anion
C)the dissociation of a strong base into hydroxide ions and a cation
D)the addition of water to ammonia to make ammonium hydroxide
E)the combination of an acid with a base to make only water
A)the combination of hydrogen ions and hydroxide ions to form water
B)the dissociation of a strong acid to hydrogen ions and an anion
C)the dissociation of a strong base into hydroxide ions and a cation
D)the addition of water to ammonia to make ammonium hydroxide
E)the combination of an acid with a base to make only water

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
5
Br∅nsted and Lowry suggested that bases be defined as proton acceptors.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the Br∅nsted-Lowry acids and bases in the following equation:
HCO3- + OH- ⇌ H2O + CO32-
A)acids HCO3-,H2O bases OH-,CO32-
B)acids OH-,CO32- bases HCO3-,H2O
C)acids H2O,OH- bases HCO3-,CO32-
D)acids HCO3-,OH- bases CO32-,H2O
E)acids H2O,CO32- bases HCO3-,OH-
HCO3- + OH- ⇌ H2O + CO32-
A)acids HCO3-,H2O bases OH-,CO32-
B)acids OH-,CO32- bases HCO3-,H2O
C)acids H2O,OH- bases HCO3-,CO32-
D)acids HCO3-,OH- bases CO32-,H2O
E)acids H2O,CO32- bases HCO3-,OH-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the Br∅nsted-Lowry acids and bases in the following equation:
HSO4- + C2H3O2- ⇌ HC2H3O2 + SO42-
A)acids HSO4-,SO42- bases HC2H3O2,C2H3O2-
B)acids HSO4-,HC2H3O2 bases SO42-,C2H3O2-
C)acids SO42-,HC2H3O2 bases HSO4-,C2H3O2-
D)acids SO42-,C2H3O2- bases HSO4-,HC2H3O2
E)acids HSO4-,C2H3O2- bases HC2H3O2,SO42-
HSO4- + C2H3O2- ⇌ HC2H3O2 + SO42-
A)acids HSO4-,SO42- bases HC2H3O2,C2H3O2-
B)acids HSO4-,HC2H3O2 bases SO42-,C2H3O2-
C)acids SO42-,HC2H3O2 bases HSO4-,C2H3O2-
D)acids SO42-,C2H3O2- bases HSO4-,HC2H3O2
E)acids HSO4-,C2H3O2- bases HC2H3O2,SO42-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
8
The definition of a neutralization reaction as a reaction in which an acid reacts with a base to produce water and a salt is inherent in:
A)only the Arrhenius theory
B)both the Arrhenius and the Br∅nsted-Lowry theories
C)only the Br∅nsted-Lowry theory
D)both the Br∅nsted-Lowry and the Lewis theories
E)only the Lewis theory
A)only the Arrhenius theory
B)both the Arrhenius and the Br∅nsted-Lowry theories
C)only the Br∅nsted-Lowry theory
D)both the Br∅nsted-Lowry and the Lewis theories
E)only the Lewis theory

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
9
H2SO4 is a weaker acid than H2SO3.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
10
When comparing binary acids of the elements in the same row of the periodic table,acid strength increases as the polarity of the element-hydrogen bond increases.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following are Br∅nsted-Lowry acids?
I.CH3COOH
II.[Cu(H2O)4]2+
III.H2O
IV.CH3NH2
V.H3O+
A)I,IIand III
B)II,III,and IV
C)I,II,IIIand IV
D)I,II,III,and V
E)II,IIIand V
I.CH3COOH
II.[Cu(H2O)4]2+
III.H2O
IV.CH3NH2
V.H3O+
A)I,IIand III
B)II,III,and IV
C)I,II,IIIand IV
D)I,II,III,and V
E)II,IIIand V

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
12
Choose the Br∅nsted-Lowry acids and bases in the following equation:
HCN + OH- ⇌ H2O + CN-
A)acids HCN,CN- bases OH-,H2O
B)acids CN-,OH- bases HCN,H2O
C)acids HCN,H2O bases OH-,CN-
D)acids OH-,H2O bases CN-,HCN
E)acids HCN,OH- bases H2O,CN-
HCN + OH- ⇌ H2O + CN-
A)acids HCN,CN- bases OH-,H2O
B)acids CN-,OH- bases HCN,H2O
C)acids HCN,H2O bases OH-,CN-
D)acids OH-,H2O bases CN-,HCN
E)acids HCN,OH- bases H2O,CN-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
13
HNO3 is a strong acid.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the Br∅nsted-Lowry acids and bases in the following equation:
NH4+ + OH- ⇌ H2O + NH3
A)acids NH4+,OH- bases H2O,NH3
B)acids OH-,H2O bases NH3,NH4+
C)acids NH4+,OH- bases NH4+,H2O
D)acids NH4+,H2O bases OH-,NH3
E)acids NH4+,NH3 bases OH-,H2O
NH4+ + OH- ⇌ H2O + NH3
A)acids NH4+,OH- bases H2O,NH3
B)acids OH-,H2O bases NH3,NH4+
C)acids NH4+,OH- bases NH4+,H2O
D)acids NH4+,H2O bases OH-,NH3
E)acids NH4+,NH3 bases OH-,H2O

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
15
In the equilibrium system described by:
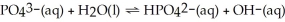
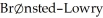 Theory would designate:
Theory would designate:
A)PO43- and H2O as the bases
B)H2O and OH- as a conjugate pair
C)HPO42- and OH- as the acids
D)HPO42- and H2O as a conjugate pair
E)PO43- as amphiprotic
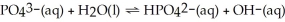
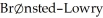 Theory would designate:
Theory would designate:A)PO43- and H2O as the bases
B)H2O and OH- as a conjugate pair
C)HPO42- and OH- as the acids
D)HPO42- and H2O as a conjugate pair
E)PO43- as amphiprotic

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
16
Amine bases are known as strong bases.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
17
According to the Arrhenius theory,a neutralization reaction involves the combination of hydrogen ions and hydroxide ions to form water.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
18
Proton acceptor is an abbreviated definition of:
A)Br∅nsted-Lowry base
B)Br∅nsted-Lowry acid
C)Lewis base
D)Lewis acid
E)Arrhenius acid
A)Br∅nsted-Lowry base
B)Br∅nsted-Lowry acid
C)Lewis base
D)Lewis acid
E)Arrhenius acid

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the Br∅nsted-Lowry acids and bases in the following equation:
H2O + NH2- ⇌ NH3 + OH-
A)acids H2O,OH- bases NH3,NH2-
B)acids NH2-,NH3 bases H2O,OH-
C)acids H2O,NH2- bases OH-,NH3
D)acids NH3,NH2- bases OH-,H2O
E)acids H2O,NH3 bases NH2-,OH-
H2O + NH2- ⇌ NH3 + OH-
A)acids H2O,OH- bases NH3,NH2-
B)acids NH2-,NH3 bases H2O,OH-
C)acids H2O,NH2- bases OH-,NH3
D)acids NH3,NH2- bases OH-,H2O
E)acids H2O,NH3 bases NH2-,OH-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
20
In the reaction BF3 + NH3 → F3B:NH3,BF3 acts as a Br∅nsted acid.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
21
A saturated aqueous solution of calcium hydroxide has a pH of 12.25.What is the [Ca2+] in such a solution?
A)0.018
B)5.6 × 10-13
C)2.3 × 10-5
D)0.035
E)8.9 × 10-3
A)0.018
B)5.6 × 10-13
C)2.3 × 10-5
D)0.035
E)8.9 × 10-3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a logical inference from the fact that a 0.10 M solution of potassium acetate,KC2H3O2,is less alkaline than a 0.10 M solution of potassium cyanide,KCN?
A)Hydrocyanic acid is a weaker acid than acetic acid.
B)Cyanides are less soluble than acetates.
C)Hydrocyanic acid is less soluble in water than acetic acid.
D)Acetic acid is a weaker acid than hydrocyanic acid.
E)0.10 M potassium acetate is more concentrated than 0.10 M potassium cyanide.
A)Hydrocyanic acid is a weaker acid than acetic acid.
B)Cyanides are less soluble than acetates.
C)Hydrocyanic acid is less soluble in water than acetic acid.
D)Acetic acid is a weaker acid than hydrocyanic acid.
E)0.10 M potassium acetate is more concentrated than 0.10 M potassium cyanide.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
23
A solution has pOH of -0.47.This means that:
A)the solution has a pH of 13.53
B)the solution has an [OH-] = 0.34 M
C)the solution has an [OH-] greater than 10.0 M
D)the solution has an [OH-] = 2.95 M
E)The solution has an [H+] = 2.95 M
A)the solution has a pH of 13.53
B)the solution has an [OH-] = 0.34 M
C)the solution has an [OH-] greater than 10.0 M
D)the solution has an [OH-] = 2.95 M
E)The solution has an [H+] = 2.95 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
24
A 0.214 M aqueous solution of a monoprotic base has a pH of 11.48.What is the ionization constant of this base?
A)1.4 × 10-2
B)4.3 × 10-5
C)1.5 × 10-11
D)5.1 × 10-23
E)2.0 × 10-6
A)1.4 × 10-2
B)4.3 × 10-5
C)1.5 × 10-11
D)5.1 × 10-23
E)2.0 × 10-6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
25
A 0.505 g sample of a monoprotic base (mm = 45.09 g/mol)was dissolved in water to produce 100.0 mL of solution with a pH = 11.84.What is the ionization constant of this base?
A)4.3 × 10-5
B)1.9 × 10-23
C)4.3 × 10-4
D)3.4 × 10-1
E)1.3 × 10-11
A)4.3 × 10-5
B)1.9 × 10-23
C)4.3 × 10-4
D)3.4 × 10-1
E)1.3 × 10-11

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
26
At 25 °C,the pH of pure water is:
A)0
B)>0,<7
C)7
D)>7,<14
E)14
A)0
B)>0,<7
C)7
D)>7,<14
E)14

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
27
A 0.632 M aqueous solution of a monoprotic base has a pH of 11.53.What is the ionization constant of this base?
A)1.8 × 10-5
B)2.1 × 10-3
C)1.6 × 10-23
D)5.0 × 10-12
E)5.3 × 10-3
A)1.8 × 10-5
B)2.1 × 10-3
C)1.6 × 10-23
D)5.0 × 10-12
E)5.3 × 10-3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
28
pOH = 3.14 is equivalent to:
A)pH = 11
B)[H+] = 1.4 × 10-10 M
C)[OH-] = 7.2 × 10-4 M
D)[H+} = 7.0 × 10-4 M
E)[OH-] = 3.14 × 10-7 M
A)pH = 11
B)[H+] = 1.4 × 10-10 M
C)[OH-] = 7.2 × 10-4 M
D)[H+} = 7.0 × 10-4 M
E)[OH-] = 3.14 × 10-7 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
29
0.653 g of a monoprotic acid (MW= 157 g/mol)is dissolved in water to produce 50.0 mL of a solution with pH = 2.13.Determine the ionization constant of the acid.
A)7.9 × 10-3
B)8.9 × 10-2
C)6.6 × 10-4
D)3.9 × 10-2
E)3.6 × 10-6
A)7.9 × 10-3
B)8.9 × 10-2
C)6.6 × 10-4
D)3.9 × 10-2
E)3.6 × 10-6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the strongest acid.
A)HF
B)H2CO3
C)HCN
D)HC2H3O2
E)HClO4
A)HF
B)H2CO3
C)HCN
D)HC2H3O2
E)HClO4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
31
The pH of a solution of NH4C2H3O2 is approximately 7.The best explanation is:
A)This salt does not react with water.
B)Ammonium acetate is a weak electrolyte.
C)All salts of weak acids and weak bases are neutral.
D)Aqueous ammonia and acetic acid have approximately equal ionization constants.
E)The salt is a product of a strong acid and a strong base.
A)This salt does not react with water.
B)Ammonium acetate is a weak electrolyte.
C)All salts of weak acids and weak bases are neutral.
D)Aqueous ammonia and acetic acid have approximately equal ionization constants.
E)The salt is a product of a strong acid and a strong base.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
32
0.272 g of a monoprotic acid (MW = 189 g/mol)is dissolved in water to produce 25.0 mL of a solution with pH = 4.93.Determine the ionization constant of the acid.
A)4.1 × 10-8
B)1.4 × 10-10
C)2.1 × 10-4
D)2.8 × 10-7
E)2.4 × 10-9
A)4.1 × 10-8
B)1.4 × 10-10
C)2.1 × 10-4
D)2.8 × 10-7
E)2.4 × 10-9

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the strongest base?
A)Cl-
B)ClO4-
C)F-
D)NO3-
E)H2O
A)Cl-
B)ClO4-
C)F-
D)NO3-
E)H2O

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
34
A 0.0925 g sample of a monoprotic base (mm = 17.03 g/mol)was dissolved in water to produce 100 mL of solution with a pH = 11.00.What is the ionization constant of this base?
A)1.8 × 10-6
B)1.8 × 10-11
C)1.8 × 10-21
D)1.8 × 10-5
E)1.1 × 10-6
A)1.8 × 10-6
B)1.8 × 10-11
C)1.8 × 10-21
D)1.8 × 10-5
E)1.1 × 10-6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
35
For which of the following polyprotic acids is the first ionization step approximately 100%?
A)H2S
B)H2SO3
C)H2CO3
D)H2SO4
E)H3PO4
A)H2S
B)H2SO3
C)H2CO3
D)H2SO4
E)H3PO4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
36
Choose the INCORRECT statement.The term pH:
A)refers to the "potential" of hydrogen ion
B)= -ln [H+]
C)= 14 - pOH
D)is more convenient than exponential notation
E)= -log [H3O+]
A)refers to the "potential" of hydrogen ion
B)= -ln [H+]
C)= 14 - pOH
D)is more convenient than exponential notation
E)= -log [H3O+]

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
37
What is the [HPO4-2] of a solution labeled "0.10 M phosphoric acid"?
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
A)4.2 × 10-13 M
B)6.3 × 10-8 M
C)7.1 × 10-3 M
D)1.6 × 10-9 M
E)1.6 × 10-16 M
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
A)4.2 × 10-13 M
B)6.3 × 10-8 M
C)7.1 × 10-3 M
D)1.6 × 10-9 M
E)1.6 × 10-16 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the reaction:
HC2H3O2 + H2O ⇌ H3O+ + C2H3O2-
Choose the pair of substances that are both bases in the reaction.
A)HC2H3O2 and H3O+
B)H2O and C2H3O2-
C)H2O and H3O+
D)HC2H3O2 and C2H3O2-
E)H3O+ and HC2H3O2
HC2H3O2 + H2O ⇌ H3O+ + C2H3O2-
Choose the pair of substances that are both bases in the reaction.
A)HC2H3O2 and H3O+
B)H2O and C2H3O2-
C)H2O and H3O+
D)HC2H3O2 and C2H3O2-
E)H3O+ and HC2H3O2

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
39
What is the [AsO43-] for a solution labeled "0.10 M arsenic acid (H3AsO4)"?
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
A)1 × 10-7 M
B)1 × 10-17 M
C)3 × 10-12 M
D)3 × 10-19 M
E)6 × 10-10 M
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
A)1 × 10-7 M
B)1 × 10-17 M
C)3 × 10-12 M
D)3 × 10-19 M
E)6 × 10-10 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
40
0.375 g of a monoprotic acid (MW = 245 g/mol)is dissolved in water to produce 25.0 mL of a solution with pH = 3.28.Determine the ionization constant of the acid.
A)8.56 × 10-3
B)4.5 × 10-6
C)7.4 × 10-5
D)4.5 × 10-3
E)2.3 × 10-2
A)8.56 × 10-3
B)4.5 × 10-6
C)7.4 × 10-5
D)4.5 × 10-3
E)2.3 × 10-2

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
41
In which of the following cases is water acting as a base under the Lewis definitions,but not under the Bronsted-Lowry definitions?
A)H2O(l)+ HF(g)⇌ H3O+(aq)+ F-(aq)
B)H2O(l)+ CN-(aq)⇌ OH-(aq)+ HCN(aq)
C)2 H2O(l)+ Cu2+(aq)⇌ Cu(H2O)22+(aq)
D)2 H2O(l)+ PO43-(aq)⇌ 2 OH-(aq)+ H2PO4-(aq)
E)2 H2O(l)[electrolysis] ⇌ 2 H2(g)+ O2(g)
A)H2O(l)+ HF(g)⇌ H3O+(aq)+ F-(aq)
B)H2O(l)+ CN-(aq)⇌ OH-(aq)+ HCN(aq)
C)2 H2O(l)+ Cu2+(aq)⇌ Cu(H2O)22+(aq)
D)2 H2O(l)+ PO43-(aq)⇌ 2 OH-(aq)+ H2PO4-(aq)
E)2 H2O(l)[electrolysis] ⇌ 2 H2(g)+ O2(g)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
42
In the following reversible reaction the Br∅nsted acids are ________.
HCO3-(aq)+ OH-(aq)⇌ CO32-(aq)+ H2O
A)HCO3- and H2O
B)HCO3- and CO32-
C)OH- and CO32-
D)OH- and H2O
E)H2O and CO32-
HCO3-(aq)+ OH-(aq)⇌ CO32-(aq)+ H2O
A)HCO3- and H2O
B)HCO3- and CO32-
C)OH- and CO32-
D)OH- and H2O
E)H2O and CO32-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
43
A 250.0 ml sample of gaseous hydrogen bromide,measured at 22.9 °C and 0.930 atm,was dissolved in sufficient pure water to form 250.0 ml of solution.What was the pH of that solution?
A)1.42
B)2.58
C)3.83
D)4.18
E)5.68
A)1.42
B)2.58
C)3.83
D)4.18
E)5.68

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
44
What is the pH of a solution prepared by dissolving 0.140 g of potassium hydroxide in sufficient pure water to prepare 250.0 ml of solution?
A)2.000
B)6.000
C)10.000
D)12.000
E)14.000
A)2.000
B)6.000
C)10.000
D)12.000
E)14.000

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
45
What would be the pH of a solution prepared by dissolving 14.4 g NaOH (MW = 40.0 g/mol)in enough water to make 1.05 L of solution?
A)13.54
B)0.46
C)13.58
D)13.66
E)7.00
A)13.54
B)0.46
C)13.58
D)13.66
E)7.00

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
46
A saturated aqueous solution of calcium hydroxide is approximately 0.13% calcium hydroxide,by mass,and has a density of 1.02 g ml-1.What is the pH of such a solution?
A)11.95
B)12.25
C)12.55
D)12.75
E)13.00
A)11.95
B)12.25
C)12.55
D)12.75
E)13.00

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following are Lewis bases?
I.BCl3
II.H-
III.H2O
IV.NH3
V.AlCl3
A)II,IIIand IV
B)I,II,and III
C)III,IVand V
D)I,II,and V
E)I,II,and IV
I.BCl3
II.H-
III.H2O
IV.NH3
V.AlCl3
A)II,IIIand IV
B)I,II,and III
C)III,IVand V
D)I,II,and V
E)I,II,and IV

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
48
For NH3,predict whether a 1 M aqueous solution is acidic,basic or neutral and why.
A)acidic because NH3 is a strong acid
B)basic because NH3 is a weak base
C)neutral because there is no hydrolysis
D)basic because NH3 is the salt of a weak acid
E)acidic because NH3 is the salt of a weak base
A)acidic because NH3 is a strong acid
B)basic because NH3 is a weak base
C)neutral because there is no hydrolysis
D)basic because NH3 is the salt of a weak acid
E)acidic because NH3 is the salt of a weak base

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
49
Complete the following equation.List the conjugate acid base pairs.Put the base first in each pair.
HCl + NH3 ⇌
A)Cl- + NH4+ (NH3,NH4+)(Cl-,HCl)
B)Cl- + NH4+ (NH3,HCl)(Cl-,NH4+)
C)H2 + Cl- + NH2- (NH3,NH2-)(Cl-,HCl)
D)H2Cl+ + NH2- (NH3,NH2-)(HCl,H2Cl)
E)Cl- + NH4+ (NH3,Cl-)(NH4+,HCl)
HCl + NH3 ⇌
A)Cl- + NH4+ (NH3,NH4+)(Cl-,HCl)
B)Cl- + NH4+ (NH3,HCl)(Cl-,NH4+)
C)H2 + Cl- + NH2- (NH3,NH2-)(Cl-,HCl)
D)H2Cl+ + NH2- (NH3,NH2-)(HCl,H2Cl)
E)Cl- + NH4+ (NH3,Cl-)(NH4+,HCl)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
50
Which species in the following reaction acts as a Lewis acid?
CuSO4(s)+ 4 NH3(aq)⇌ [Cu(NH3)4]2+(aq)+ SO42-(aq)
A)SO42-
B)Cu2+
C)[Cu(NH3)4]2+(aq)
D)NH3
E)[Cu(NH3)4]2+(aq)and SO42-
CuSO4(s)+ 4 NH3(aq)⇌ [Cu(NH3)4]2+(aq)+ SO42-(aq)
A)SO42-
B)Cu2+
C)[Cu(NH3)4]2+(aq)
D)NH3
E)[Cu(NH3)4]2+(aq)and SO42-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
51
The concept of an acid not limited to H+ or species containing one or more protons is inherent in:
A)only the Arrhenius theory
B)both the Arrhenius and the Br∅nsted-Lowry theories
C)only the Br∅nsted-Lowry theory
D)both the Br∅nsted-Lowry and the Lewis theories
E)only the Lewis theory
A)only the Arrhenius theory
B)both the Arrhenius and the Br∅nsted-Lowry theories
C)only the Br∅nsted-Lowry theory
D)both the Br∅nsted-Lowry and the Lewis theories
E)only the Lewis theory

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements concerning aqueous solutions of salts is FALSE?
A)For any salt of a strong acid and a strong base,the pH will be about 7.
B)For any salt of a strong acid and a weak base,the pH will be <7.
C)For any salt of a weak acid and a strong base,the pH will be >7.
D)For any salt of a weak acid and a weak base,the pH will be about 7.
E)Salt solutions can have a pH of 7.
A)For any salt of a strong acid and a strong base,the pH will be about 7.
B)For any salt of a strong acid and a weak base,the pH will be <7.
C)For any salt of a weak acid and a strong base,the pH will be >7.
D)For any salt of a weak acid and a weak base,the pH will be about 7.
E)Salt solutions can have a pH of 7.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
53
If one mole of Ba(OH)2 is added to enough water to make 10 liters of solution,the pH of the resulting solution is ________.
A)13.3
B)1.0
C)0.7
D)13.0
E)12.5
A)13.3
B)1.0
C)0.7
D)13.0
E)12.5

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
54
Which indication of relative acid strengths is INCORRECT?
A)HCl > HF
B)HClO2 > HClO
C)H2SO4 > H2SO3
D)H2SO3 > HNO3
E)CH3CO2H > CH3CH2OH
A)HCl > HF
B)HClO2 > HClO
C)H2SO4 > H2SO3
D)H2SO3 > HNO3
E)CH3CO2H > CH3CH2OH

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
55
What is the [OH-] of a solution prepared by dissolving 0.0912 g of hydrogen chloride in sufficient pure water to prepare 250.0 ml of solution?
A)1.00 × 10-2 M
B)1.00 × 10-8 M
C)1.00 × 10-12 M
D)1.00 × 10-4 M
E)1.0 M
A)1.00 × 10-2 M
B)1.00 × 10-8 M
C)1.00 × 10-12 M
D)1.00 × 10-4 M
E)1.0 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
56
What is the [Cl-] of a solution prepared by dissolving 0.1824 g of hydrogen chloride in sufficient pure water to prepare 500.0 ml of solution?
A)1.00 × 10-2 M
B)1.00 × 10-8 M
C)1.00 × 10-12 M
D)1.00 × 10-4 M
E)1.0 M
A)1.00 × 10-2 M
B)1.00 × 10-8 M
C)1.00 × 10-12 M
D)1.00 × 10-4 M
E)1.0 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
57
For HI,predict whether the solution is acidic,basic or neutral and why.
A)acidic because HI is a strong acid
B)basic because HI is a weak base
C)neutral because there is no hydrolysis
D)basic because HI is the salt of a weak acid
E)acidic because HI is the salt of a weak base
A)acidic because HI is a strong acid
B)basic because HI is a weak base
C)neutral because there is no hydrolysis
D)basic because HI is the salt of a weak acid
E)acidic because HI is the salt of a weak base

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
58
What is the pH of a 0.470 M aqueous solution of pyridine?
Kb = 1.5 × 10-9
A)9.0
B)11.2
C)2.8
D)9.4
E)4.6
Kb = 1.5 × 10-9
A)9.0
B)11.2
C)2.8
D)9.4
E)4.6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
59
What is the [K+] of a solution prepared by dissolving 0.140 g of potassium hydroxide in sufficient pure water to prepare 250.0 ml of solution?
A)10-5 M
B)10-8 M
C)10-12 M
D)10-4 M
E)10-2 M
A)10-5 M
B)10-8 M
C)10-12 M
D)10-4 M
E)10-2 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
60
In the reaction BF3 + NH3 ⇌ F3B:NH3,BF3 acts as:
A)an Arrhenius base
B)a Lewis base
C)a Br∅nsted acid
D)a Lewis acid
E)an Arrhenius acid
A)an Arrhenius base
B)a Lewis base
C)a Br∅nsted acid
D)a Lewis acid
E)an Arrhenius acid

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
61
What is the [H2AsO4-] for an aqueous solution labeled "0.10 M arsenic acid (H3AsO4)"?
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
A)1 × 10-7 M
B)6 × 10-3 M
C)0.02 M
D)0.08 M
E)6 × 10-10 M
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
A)1 × 10-7 M
B)6 × 10-3 M
C)0.02 M
D)0.08 M
E)6 × 10-10 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
62
For NH4Cl,predict whether the aqueous solution is acidic,basic or neutral and why.
A)acidic because NH4Cl is a strong acid
B)basic because NH4Cl is a weak base
C)neutral because there is no hydrolysis
D)basic because NH4Cl is the salt of a weak acid
E)acidic because NH4Cl is the salt of a weak base
A)acidic because NH4Cl is a strong acid
B)basic because NH4Cl is a weak base
C)neutral because there is no hydrolysis
D)basic because NH4Cl is the salt of a weak acid
E)acidic because NH4Cl is the salt of a weak base

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
63
What is the pH of a 0.052 M aqueous solution of sodium acetate? Ka (acetic acid)= 1.8 × 10-5
A)10.0
B)5.3
C)11.0
D)8.7
E)3.0
A)10.0
B)5.3
C)11.0
D)8.7
E)3.0

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
64
For NaC2H3O2,predict whether the aqueous solution is acidic,basic or neutral and why.
A)acidic because NaC2H3O2 is a strong acid
B)basic because NaC2H3O2 is a weak base
C)neutral because there is no hydrolysis
D)basic because NaC2H3O2 is the salt of a weak acid
E)acidic because NaC2H3O2 is the salt of a weak base
A)acidic because NaC2H3O2 is a strong acid
B)basic because NaC2H3O2 is a weak base
C)neutral because there is no hydrolysis
D)basic because NaC2H3O2 is the salt of a weak acid
E)acidic because NaC2H3O2 is the salt of a weak base

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
65
What is the pH of an aqueous solution labeled "0.10 M phosphoric acid"?
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
A)5.7
B)4.3
C)3.1
D)2.3
E)1.6
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
A)5.7
B)4.3
C)3.1
D)2.3
E)1.6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
66
What is the concentration of free sulfate ion in an aqueous solution labeled "3.6 M H2SO4"?
[Ka2 = 1.1 × 10-2]
A)0.011 M
B)0.040 M
C)0.20 M
D)0.60 M
E)1.8 M
[Ka2 = 1.1 × 10-2]
A)0.011 M
B)0.040 M
C)0.20 M
D)0.60 M
E)1.8 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
67
What is the pH of a 0.250 M aqueous solution of formic acid?
Ka = 1.8 × 10-4
A)11.8
B)2.2
C)0.60
D)5.4
E)8.6
Ka = 1.8 × 10-4
A)11.8
B)2.2
C)0.60
D)5.4
E)8.6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
68
What is the pH for a solution labeled "0.10 M arsenic acid (H3AsO4)"?
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
A)1.6
B)4.0
C)6.3
D)1.0
E)3.2
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
A)1.6
B)4.0
C)6.3
D)1.0
E)3.2

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
69
A certain acid,HA,has a Ka given by:
HA + H2O ⇌ H3O+ + A- Ka = 6.80 × 10-6
What is the pH of a 0.247 M aqueous solution of the acid's potassium salt,KA,which undergoes the hydrolysis reaction?
A- + H2O ⇌ OH- + HA
A)4.72
B)9.28
C)4.11
D)9.89
E)9.44
HA + H2O ⇌ H3O+ + A- Ka = 6.80 × 10-6
What is the pH of a 0.247 M aqueous solution of the acid's potassium salt,KA,which undergoes the hydrolysis reaction?
A- + H2O ⇌ OH- + HA
A)4.72
B)9.28
C)4.11
D)9.89
E)9.44

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
70
What is the pH of an aqueous solution labeled "0.50 M sodium fluoride" if the ionization constant of hydrofluoric acid is 6.0 × 10-4?
A)10.8
B)8.5
C)7.1
D)6.9
E)5.5
A)10.8
B)8.5
C)7.1
D)6.9
E)5.5

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
71
What is the pH of a 0.570 M aqueous solution of aniline?
Kb = 7.4 × 10-10
A)11.4
B)2.6
C)9.8
D)4.7
E)9.3
Kb = 7.4 × 10-10
A)11.4
B)2.6
C)9.8
D)4.7
E)9.3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
72
What is the pH of a 0.375 M aqueous solution of benzoic acid?
Ka = 6.3 × 10-5
A)8.9
B)5.1
C)2.3
D)0.43
E)11.7
Ka = 6.3 × 10-5
A)8.9
B)5.1
C)2.3
D)0.43
E)11.7

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
73
What is the pH of a 0.380 M aqueous solution of ethylamine?
Kb = 4.3 × 10-4
A)12.1
B)1.9
C)10.2
D)5.5
E)8.5
Kb = 4.3 × 10-4
A)12.1
B)1.9
C)10.2
D)5.5
E)8.5

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
74
The ionization constant for ammonia is 1.8 × 10-5.What is the pH of an aqueous solution labeled 0.50 M ammonia?
A)7.30
B)9.12
C)10.26
D)11.48
E)12.52
A)7.30
B)9.12
C)10.26
D)11.48
E)12.52

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
75
For NaCl,predict whether the aqueous solution is acidic,basic or neutral and why.
A)acidic because NaCl is a strong acid
B)basic because NaCl is a weak base
C)neutral because there is no hydrolysis
D)basic because NaCl is the salt of a weak acid
E)acidic because NaCl is the salt of a weak base
A)acidic because NaCl is a strong acid
B)basic because NaCl is a weak base
C)neutral because there is no hydrolysis
D)basic because NaCl is the salt of a weak acid
E)acidic because NaCl is the salt of a weak base

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
76
For Na2CO3,predict whether the aqueous solution is acidic,basic or neutral and why.
A)acidic because Na2CO3 is a strong acid
B)basic because Na2CO3 is a weak base
C)neutral because there is no hydrolysis
D)basic because Na2CO3 is the salt of a weak acid
E)acidic because Na2CO3 is the salt of a weak base
A)acidic because Na2CO3 is a strong acid
B)basic because Na2CO3 is a weak base
C)neutral because there is no hydrolysis
D)basic because Na2CO3 is the salt of a weak acid
E)acidic because Na2CO3 is the salt of a weak base

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
77
Hypochlorous acid (HOCl)has an ionization constant of 3.2 × 10-8.What is its percent ionization in 1.0 M and 0.10 M solutions,respectively?
A)0.018% and 0.057%
B)0.032% and 0.0032%
C)0.57% and 0.18%
D)0.57% in both
E)0.32% in both
A)0.018% and 0.057%
B)0.032% and 0.0032%
C)0.57% and 0.18%
D)0.57% in both
E)0.32% in both

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
78
The Kb value for methylamine is 4.2 × 10-4.What is the pH of an aqueous solution for which the label reads "0.042 M CH3NH2"?
A)2.4
B)4.8
C)9.2
D)11.6
E)12.3
A)2.4
B)4.8
C)9.2
D)11.6
E)12.3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
79
What is the pH of a 0.530 M aqueous solution of hypochlorus acid?
Ka = 2.9 × 10-8
A)10.6
B)3.4
C)10.1
D)3.9
E)0.28
Ka = 2.9 × 10-8
A)10.6
B)3.4
C)10.1
D)3.9
E)0.28

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
80
What is the [H2PO4-] of an aqueous solution labeled "0.10 M phosphoric acid"?
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
A)0.023 M
B)6.3 × 10-8 M
C)7.1 × 10-3 M
D)4.2 × 10-13 M
E)0.013 M
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
A)0.023 M
B)6.3 × 10-8 M
C)7.1 × 10-3 M
D)4.2 × 10-13 M
E)0.013 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck


