Deck 5: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/154
Play
Full screen (f)
Deck 5: Gases
1
A gas occupies 3.33 L at 2.23 bar. What is the volume at 2.50 bar?
A)1.67 L
B)3.73 L
C)2.97 L
D)0.268 L
E)18.6 L
A)1.67 L
B)3.73 L
C)2.97 L
D)0.268 L
E)18.6 L
2.97 L
2
To what volume will a sample of gas expand if it is heated from 50.0 °C and 2.33 L to 500.0 °C?
A)5.58 L
B)9.74 L
C)10.3 L
D)17.9 L
E)4.38 L
A)5.58 L
B)9.74 L
C)10.3 L
D)17.9 L
E)4.38 L
5.58 L
3
The atmospheric pressure is 715 mmHg. What is the pressure in bar?
A)1.10 bar
B)0.940 bar
C)1.08 bar
D)0.9533 bar
E)0.9197 bar
A)1.10 bar
B)0.940 bar
C)1.08 bar
D)0.9533 bar
E)0.9197 bar
0.9533 bar
4
What volume will a balloon occupy at 1.01 bar if the balloon has a volume of 7.6 L at 3.85 bar?
A)2.0 L
B)5.0 L
C)29 L
D)35 L
E)17 L
A)2.0 L
B)5.0 L
C)29 L
D)35 L
E)17 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
5
A large balloon is initially filled to a volume of 25.0 L at 353 K and a pressure of 2575 mmHg. What volume of gas will the balloon contain at 1.368 bar and 253 K?
A)22.2 L
B)87.5 L
C)11.4 L
D)45.0 L
E)58.6 L
A)22.2 L
B)87.5 L
C)11.4 L
D)45.0 L
E)58.6 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
6
A syringe contains 0.65 moles of He gas that occupy 750.0 mL. What volume (in L)of gas will the syringe hold if 0.35 moles of Ne is added?
A)0.87 L
B)4.9 L
C)1.2 L
D)2.1 L
E)1.9 L
A)0.87 L
B)4.9 L
C)1.2 L
D)2.1 L
E)1.9 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
7
The volume of a gas is inversely proportional to the pressure of a gas. What is this known as?
A)Avogadro's law
B)Ideal gas law
C)Charles's law
D)Boyle's law
E)Dalton's law
A)Avogadro's law
B)Ideal gas law
C)Charles's law
D)Boyle's law
E)Dalton's law

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
8
If a sample of 0.29 moles of Ar occupies 3.8 L under certain conditions, what volume will 0.66 moles occupy under the same conditions?
A)12 L
B)8.6 L
C)17 L
D)5.0 L
E)15 L
A)12 L
B)8.6 L
C)17 L
D)5.0 L
E)15 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
9
Convert 717.28 mmHg to bar.
A)1.046 bar
B)0.9438 bar
C)1.060 bar
D)0.9563 bar
E)0.8971 bar
A)1.046 bar
B)0.9438 bar
C)1.060 bar
D)0.9563 bar
E)0.8971 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
10
Convert 3.1473 atm to bar.
A)4.976 bar
B)3.189 bar
C)2.577 bar
D)4.112 bar
E)3.875 bar
A)4.976 bar
B)3.189 bar
C)2.577 bar
D)4.112 bar
E)3.875 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
11
The volume of a gas is proportional to number of moles of a gas. What is this known as?
A)Avogadro's law
B)Ideal gas law
C)Charles's law
D)Boyle's law
E)Dalton's law
A)Avogadro's law
B)Ideal gas law
C)Charles's law
D)Boyle's law
E)Dalton's law

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
12
Convert 4744.1 mmHg to bar.
A)7.549 bar
B)5.2423 bar
C)5.278 bar
D)6.817 bar
E)6.325 bar
A)7.549 bar
B)5.2423 bar
C)5.278 bar
D)6.817 bar
E)6.325 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
13
To what temperature must a balloon, initially at 25 °C and 2.00 L, be heated to have a volume of 6.00 L?
A)993 K
B)403 K
C)75 K
D)655 K
E)894 K
A)993 K
B)403 K
C)75 K
D)655 K
E)894 K

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
14
A gas is at 35.0 °C and 4.50 L. What is the temperature at 9.00 L?
A)343°C
B)70.0°C
C)616°C
D)1.16°C
E)17.5°C
A)343°C
B)70.0°C
C)616°C
D)1.16°C
E)17.5°C

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
15
What volume (in mL)will a sample of F2 gas occupy in a syringe at 5.57 bar if the F2 has a volume of 25.0 mL at 1.22 bar?
A)11 mL
B)17 mL
C)3.8 mL
D)5.5 mL
E)7.6 mL
A)11 mL
B)17 mL
C)3.8 mL
D)5.5 mL
E)7.6 mL

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
16
Convert 123.80 kPa to bar.
A)1.222 bar
B)1.087 bar
C)0.8078 bar
D)0.9186 bar
E)1.238 bar
A)1.222 bar
B)1.087 bar
C)0.8078 bar
D)0.9186 bar
E)1.238 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
17
Convert 29.592 inches Hg to bar.
A)0.9889 bar
B)1.011 bar
C)1.002 bar
D)1.023 bar
E)0.9980 bar
A)0.9889 bar
B)1.011 bar
C)1.002 bar
D)1.023 bar
E)0.9980 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
18
The volume of a gas is proportional to the temperature of a gas. What is this known as?
A)Avogadro's law
B)Ideal gas law
C)Charles's law
D)Boyle's law
E)Dalton's law
A)Avogadro's law
B)Ideal gas law
C)Charles's law
D)Boyle's law
E)Dalton's law

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
19
Convert 1.25 atm to bar.
A)1.23 bar
B)0.975 bar
C)1.27 bar
D)0.7874 bar
E)1.25 bar
A)1.23 bar
B)0.975 bar
C)1.27 bar
D)0.7874 bar
E)1.25 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
20
A sample of 0.300 moles of nitrogen occupies 0.600 L. Under the same conditions, what number of moles occupies 1.200 L?
A)0.600 moles
B)1.50 moles
C)0.33 moles
D)6.00 moles
A)0.600 moles
B)1.50 moles
C)0.33 moles
D)6.00 moles

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
21
A syringe initially holds a sample of gas with a volume of 285 mL at 355 K and 1.88 bar. To what temperature must the gas in the syringe be heated/cooled to have a volume of 435 mL at 2.50 bar?
A)139 K
B)572 K
C)175 K
D)466 K
E)720 K
A)139 K
B)572 K
C)175 K
D)466 K
E)720 K

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
22
What pressure (in bar)will 0.44 moles of CO2 exert in a 2.6 L container at 25 °C?
A)0.35 bar
B)4.2 bar
C)4.7 bar
D)8.6 bar
E)3.6 bar
A)0.35 bar
B)4.2 bar
C)4.7 bar
D)8.6 bar
E)3.6 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
23
What mass of NO2 is contained in a 13.0 L tank at 4.58 bar and 385 K?
A)18.8 g
B)53.1 g
C)24.4 g
D)85.6 g
E)69.2 g
A)18.8 g
B)53.1 g
C)24.4 g
D)85.6 g
E)69.2 g

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
24
What pressure will 14.0 g of CO exert in a 3.5 L container at 75 °C?
A)4.1 bar
B)5.0 bar
C)6.4 bar
D)1.1 bar
E)2.3 bar
A)4.1 bar
B)5.0 bar
C)6.4 bar
D)1.1 bar
E)2.3 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
25
If 0.3781 g of a gas with a molecular weight of 18.015 g mol-1 is in a ridged 5.00 L container at 384 K, calculate the final pressure. Assume ideal behaviour.
A)0.682 bar
B)0.109 bar
C)0.0867 bar
D)0.134 bar
E)0.0963 bar
A)0.682 bar
B)0.109 bar
C)0.0867 bar
D)0.134 bar
E)0.0963 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
26
What volume would 1.02 moles of Kr occupy at a pressure of 1.65 bar and a temperature of 281.2 K?
A)28.7 L
B)14.5 L
C)12.7 L
D)6.87 L
E)16.9 L
A)28.7 L
B)14.5 L
C)12.7 L
D)6.87 L
E)16.9 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the mass of nitrogen dioxide, NO2, required if a 5.00 L container is to be filled to 0.6478 bar at a temperature of 1.00 °C.
A)5.40 g
B)8.14 g
C)3.97 g
D)5.09 g
E)6.54 g
A)5.40 g
B)8.14 g
C)3.97 g
D)5.09 g
E)6.54 g

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of an ideal gas are required to produce a pressure of 3.00 bar in a 1.50 L container with a temperature of 25.0 °C?
A)0.146
B)0.0874
C)0.182
D)0.914
E)1.08
A)0.146
B)0.0874
C)0.182
D)0.914
E)1.08

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
29
If 0.24 mol of He were added to a balloon, how large would the balloon grow? Atmospheric pressure is 0.9871 bar and the ambient temperature is 23.2 °C.
A)5.99 L
B)6.54 L
C)3.73 L
D)6.49 L
E)7.28 L
A)5.99 L
B)6.54 L
C)3.73 L
D)6.49 L
E)7.28 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
30
How many moles of CO are contained in a 5.00 L tank at 155 °C and 2.80 bar?
A)0.393 moles
B)1.10 moles
C)2.51 moles
D)0.455 moles
E)0.289 moles
A)0.393 moles
B)1.10 moles
C)2.51 moles
D)0.455 moles
E)0.289 moles

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
31
An unknown ideal gas was added to an evacuated 775 mL container at a temperature of 267.2 K. If 0.381 moles were added, what is the final pressure?
A)15.8 bar
B)12.4 bar
C)9.92 bar
D)10.9 bar
E)8.92 bar
A)15.8 bar
B)12.4 bar
C)9.92 bar
D)10.9 bar
E)8.92 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
32
A sample of gas initially has a volume of 859 mL at 565 K and 2.20 bar. What pressure will the sample have if the volume changes to 268 mL while the temperature is increased to 815 K?
A)10.2 bar
B)9.83 bar
C)15.3 bar
D)6.53 bar
E)1.05 bar
A)10.2 bar
B)9.83 bar
C)15.3 bar
D)6.53 bar
E)1.05 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the temperature, in K, of 2.20 moles of gas occupying 3.50 L at 3.30 bar.
A)63.1 K
B)5.25 K
C)337 K
D)28.0 K
A)63.1 K
B)5.25 K
C)337 K
D)28.0 K

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
34
How many moles of molecular oxygen are required to produce a pressure of 0.413 bar in a 650 mL container with a temperature of 245 K?
A)0.0132
B)0.199
C)0.00872
D)0.00971
E)0.0245
A)0.0132
B)0.199
C)0.00872
D)0.00971
E)0.0245

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the temperature if 0.0139 moles of Ne has a pressure of 1.237 bar in a 250 mL container.
A)260 °C
B)61.8 °C
C)-23.8 °C
D)15.4 °C
E)-5.57 °C
A)260 °C
B)61.8 °C
C)-23.8 °C
D)15.4 °C
E)-5.57 °C

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
36
What is the volume of 9.783 × 1023 atoms of He at 9.25 bar and 512 K?
A)7.48 L
B)3.69 L
C)1.85 L
D)15.4 L
E)30.8 L
A)7.48 L
B)3.69 L
C)1.85 L
D)15.4 L
E)30.8 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
37
What pressure will 2.6 × 1023 molecules of H2 exert in a 3.9 L container at 45 °C?
A)5.7 bar
B)1.7 bar
C)2.9 bar
D)3.4 bar
E)4.6 bar
A)5.7 bar
B)1.7 bar
C)2.9 bar
D)3.4 bar
E)4.6 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
38
What is the volume of 5.60 g of O2 at 7.78 bar and 415 K?
A)1.53 L
B)565 L
C)24.5 L
D)25.0 L
E)0.776 L
A)1.53 L
B)565 L
C)24.5 L
D)25.0 L
E)0.776 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the temperature if 3.120 moles of He has a pressure of 2.45 bar in a 23.8 L container.
A)218 K
B)314 K
C)225 K
D)334 K
E)287 K
A)218 K
B)314 K
C)225 K
D)334 K
E)287 K

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
40
How many molecules of CO2 are contained in a 10.0 L tank at 7.53 bar and 485 K?
A)1.89 × 1024 molecules
B)1.12 × 1024 molecules
C)8.32 × 1024 molecules
D)4.89 × 1024 molecules
E)3.63 × 1024 molecules
A)1.89 × 1024 molecules
B)1.12 × 1024 molecules
C)8.32 × 1024 molecules
D)4.89 × 1024 molecules
E)3.63 × 1024 molecules

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
41
What volume will 4.91 × 1022 atoms of Ne occupy at STP?
A)1.10 L
B)2.00 L
C)2.24 L
D)3.11 L
E)1.85 L
A)1.10 L
B)2.00 L
C)2.24 L
D)3.11 L
E)1.85 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following samples will have the lowest pressure if they are all at the same temperature and in identical containers (same volume)?
A)15 g F2
B)15 g Ne
C)15 g Kr
D)15 g CO2
E)All of these samples will have the same pressure.
A)15 g F2
B)15 g Ne
C)15 g Kr
D)15 g CO2
E)All of these samples will have the same pressure.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
43
A compound is found to be 30.45% N and 69.55 % O by mass. If 1.63 g of this compound occupy 389 mL at 0.00 °C and 1.0332 bar, what is the molecular formula of the compound?
A)NO2
B)N2O
C)N4O2
D)N2O5
E)N2O4
A)NO2
B)N2O
C)N4O2
D)N2O5
E)N2O4

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following gas samples would be most likely to behave ideally under the stated conditions?
A)Ne at STP
B)CO at 200 bar and 25 °C
C)SO2 at 2 bar and 0 K
D)N2 at 1 bar and -70 °C
E)O2 at 400 bar and 25 °C
A)Ne at STP
B)CO at 200 bar and 25 °C
C)SO2 at 2 bar and 0 K
D)N2 at 1 bar and -70 °C
E)O2 at 400 bar and 25 °C

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
45
A 0.465 g sample of an unknown compound occupies 245 mL at 298 K and 1.24 bar. What is the molar mass of the unknown compound?
A)26.3 g mol-1
B)33.9 g mol-1
C)12.2 g mol-1
D)38.0 g mol-1
E)81.8 g mol-1
A)26.3 g mol-1
B)33.9 g mol-1
C)12.2 g mol-1
D)38.0 g mol-1
E)81.8 g mol-1

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following samples has the greatest density at STP?
A)NO2
B)Xe
C)SO2
D)SF6
E)All of these samples have the same density at STP.
A)NO2
B)Xe
C)SO2
D)SF6
E)All of these samples have the same density at STP.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
47
The density of a gas is 1.146 g L-1 at STP. What is the gas?
A)He
B)O2
C)C2H2
D)O3
A)He
B)O2
C)C2H2
D)O3

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the density of CO2 gas at STP.
A)1.94 g L-1
B)1.80 g L-1
C)2.24 g L-1
D)4.46 g L-1
E)5.10 g L-1
A)1.94 g L-1
B)1.80 g L-1
C)2.24 g L-1
D)4.46 g L-1
E)5.10 g L-1

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the density of CO2 gas at 278 K and 1.00 bar.
A)1.14 g L-1
B)2.37 g L-1
C)1.90 g L-1
D)3.08 g L-1
E)2.11 g L-1
A)1.14 g L-1
B)2.37 g L-1
C)1.90 g L-1
D)3.08 g L-1
E)2.11 g L-1

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
50
What volume will 0.780 moles of He occupy at STP?
A)22.4 L
B)70.0 L
C)43.7 L
D)17.7 L
E)15.6 L
A)22.4 L
B)70.0 L
C)43.7 L
D)17.7 L
E)15.6 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
51
The density of a gas is 3.12 g L-1 at STP. What is the gas?
A)Cl2
B)O3
C)O2
D)F2
A)Cl2
B)O3
C)O2
D)F2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following will cause the volume of an ideal gas to triple in value?
A)raising the temperature from 25 °C to 75 °C at constant pressure
B)lowering the absolute temperature by a factor of 3 at constant pressure
C)raising the absolute temperature by a factor of 3 while increasing the pressure by a factor of 3
D)lowering the absolute temperature by a factor of 3 while increasing the pressure by a factor of 3
E)lowering the pressure by a factor of 3 while the temperature stays constant
A)raising the temperature from 25 °C to 75 °C at constant pressure
B)lowering the absolute temperature by a factor of 3 at constant pressure
C)raising the absolute temperature by a factor of 3 while increasing the pressure by a factor of 3
D)lowering the absolute temperature by a factor of 3 while increasing the pressure by a factor of 3
E)lowering the pressure by a factor of 3 while the temperature stays constant

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
53
Using the graph below, determine the gas that has the lowest density at STP. 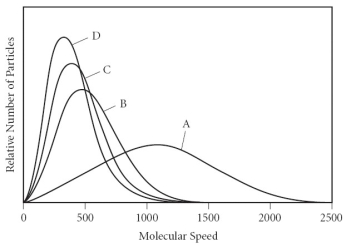
A)A
B)B
C)C
D)D
E)All of the gases have the same density at STP.

A)A
B)B
C)C
D)D
E)All of the gases have the same density at STP.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
54
The density of a gas is 1.41 g L-1 at STP. What is the gas?
A)Cl2
B)S
C)O2
D)Ne
A)Cl2
B)S
C)O2
D)Ne

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
55
Determine the density of NH3 gas at 435 K and 1.00 bar.
A)2.10 g L-1
B)0.471 g L-1
C)0.321 g L-1
D)2.24 g L-1
E)0.851 g L-1
A)2.10 g L-1
B)0.471 g L-1
C)0.321 g L-1
D)2.24 g L-1
E)0.851 g L-1

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
56
Place the following gases in order of increasing density at STP. N2 NH3 N2O4 Ar
A)N2O4 < Ar < N2 < NH3
B)Ar < N2O4 < N2 < NH3
C)N2 < Ar < N2O4 < NH3
D)NH3 < N2 < Ar < N2O4
E)Ar < N2 < NH3 < N2O4
A)N2O4 < Ar < N2 < NH3
B)Ar < N2O4 < N2 < NH3
C)N2 < Ar < N2O4 < NH3
D)NH3 < N2 < Ar < N2O4
E)Ar < N2 < NH3 < N2O4

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
57
Give the temperature and pressure at STP.
A)0° C and 1.00 bar
B)0 K and 1.00 bar
C)25° C and 30.00 in Hg
D)300 K and 1 Torr Hg
E)0° C and 1 mmHg
A)0° C and 1.00 bar
B)0 K and 1.00 bar
C)25° C and 30.00 in Hg
D)300 K and 1 Torr Hg
E)0° C and 1 mmHg

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following samples will have the greatest volume at STP?
A)22 g CO
B)22 g He
C)22 g O2
D)22 g Cl2
E)All of these samples would have the same volume at STP.
A)22 g CO
B)22 g He
C)22 g O2
D)22 g Cl2
E)All of these samples would have the same volume at STP.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
59
A 0.334 g sample of an unknown halogen occupies 109 mL at 398 K and 1.43 bar. What is the identity of the halogen?
A)Br2
B)F2
C)Cl2
D)I2
E)Ge
A)Br2
B)F2
C)Cl2
D)I2
E)Ge

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
60
Determine the density of O3 gas at 341 K and 2.14 bar.
A)3.62 g L-1
B)2.91 g L-1
C)0.321 g L-1
D)4.82 g L-1
E)3.17 g L-1
A)3.62 g L-1
B)2.91 g L-1
C)0.321 g L-1
D)4.82 g L-1
E)3.17 g L-1

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
61
Identify the gas that is the lowest percent by volume in dry air.
A)CO2
B)Ar
C)O2
D)N2
A)CO2
B)Ar
C)O2
D)N2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
62
A gas mixture contains CO, Ar, and H2. What is the total pressure of the mixture if the mole fraction of H2 is 0.35 and the pressure of H2 is 0.58 bar?
A)1.7 bar
B)0.20 bar
C)0.49 bar
D)0.60 bar
E)2.1 bar
A)1.7 bar
B)0.20 bar
C)0.49 bar
D)0.60 bar
E)2.1 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
63
What pressure would a gas mixture in a 10.0 L tank exert if it were composed of 48.5 g He and 94.6 g CO2 at 398 K?
A)7.11 bar
B)39.6 bar
C)40.1 bar
D)47.2 bar
E)58.7 bar
A)7.11 bar
B)39.6 bar
C)40.1 bar
D)47.2 bar
E)58.7 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
64
How many grams of zinc metal are required to produce 2.00 L of hydrogen gas at STP according to the chemical equation shown below? Zn(s)+ 2HCl(aq)→ ZnCl2(aq)+ H2(g)
A)0.171 g
B)5.76 g
C)11.7 g
D)131 g
A)0.171 g
B)5.76 g
C)11.7 g
D)131 g

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
65
A gas mixture consists of N2, O2, and Ne, where the mole fraction of N2 is 0.55 and the mole fraction of Ne is 0.25. If the mixture is at STP in a 5.0 L container, how many molecules of O2 are present?
A)4.5 × 1022 molecules O2
B)2.7 × 1022 molecules O2
C)3.7 × 1023 molecules O2
D)1.1 × 1023 molecules O2
E)9.3 × 1024 molecules O2
A)4.5 × 1022 molecules O2
B)2.7 × 1022 molecules O2
C)3.7 × 1023 molecules O2
D)1.1 × 1023 molecules O2
E)9.3 × 1024 molecules O2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
66
Define hypoxia.
A)oxygen starvation
B)increased oxygen concentration in body tissues
C)increased nitrogen concentration in body tissues and fluids
D)nitrogen starvation
A)oxygen starvation
B)increased oxygen concentration in body tissues
C)increased nitrogen concentration in body tissues and fluids
D)nitrogen starvation

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
67
A mixture of 10.0 g of Ne and 10.0 g Ar have a total pressure of 1.6 bar. What is the partial pressure of Ne?
A)1.1 bar
B)0.80 bar
C)0.54 bar
D)0.40 bar
E)1.3 bar
A)1.1 bar
B)0.80 bar
C)0.54 bar
D)0.40 bar
E)1.3 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
68
A mixture of 0.220 moles CO, 0.350 moles H2, and 0.640 moles He has a total pressure of 2.989 bar. What is the pressure of H2?
A)1.17 bar
B)0.865 bar
C)1.03 bar
D)0.969 bar
E)0.649 bar
A)1.17 bar
B)0.865 bar
C)1.03 bar
D)0.969 bar
E)0.649 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
69
In a container containing CO, H2, and O2, what is the mole fraction of CO if the H2 mole fraction is 0.22 and the O2 mole fraction is 0.58?
A)0.20
B)0.30
C)0.10
D)0.50
A)0.20
B)0.30
C)0.10
D)0.50

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
70
A mixture of N2, O2, and Ar has mole fractions of 0.25, 0.65, and 0.10, respectively. What is the pressure of N2 if the total pressure of the mixture is 3.9 bar?
A)2.5 bar
B)0.39 bar
C)0.67 bar
D)0.98 bar
E)1.33 bar
A)2.5 bar
B)0.39 bar
C)0.67 bar
D)0.98 bar
E)1.33 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
71
A mixture of 0.220 moles CO, 0.350 moles H2, and 0.640 moles He has a total pressure of 2.95 bar. What is the pressure of CO?
A)1.86 bar
B)0.649 bar
C)0.536 bar
D)1.54 bar
E)0.955 bar
A)1.86 bar
B)0.649 bar
C)0.536 bar
D)1.54 bar
E)0.955 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
72
The following reaction is used to generate hydrogen gas in the laboratory. If 243 mL of gas is collected at 25 °C and has a total pressure of 0.9933 bar, what mass of hydrogen is produced? A possibly useful table of water vapour pressures is provided below. Mg(s)+ 2HCl(aq)→ MgCl2(aq)+ H2(g) T (°C)P (bar)
20 0.023398
25 0.031704
30 0.042477
A)0.0196 g H2
B)0.0717 g H2
C)0.0190 g H2
D)0.0144 g H2
E)0.0449 g H2
20 0.023398
25 0.031704
30 0.042477
A)0.0196 g H2
B)0.0717 g H2
C)0.0190 g H2
D)0.0144 g H2
E)0.0449 g H2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
73
How many litres of oxygen are needed to exactly react with 19.8 g of methane at STP? CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(l)
A)13.9 L
B)27.8 L
C)56.0 L
D)60.5 L
A)13.9 L
B)27.8 L
C)56.0 L
D)60.5 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
74
Determine the volume of H2S (at 375 K and 1.216 bar)needed to produce 55.0 g of S. Assume that there is excess SO2 present. 2H2S(g)+ SO2(g)→ 3S(s)+ 2H2O(g)
A)44.0 L
B)29.3 L
C)22.7 L
D)34.1 L
E)66.0 L
A)44.0 L
B)29.3 L
C)22.7 L
D)34.1 L
E)66.0 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
75
Define the vapour pressure of water.
A)partial pressure of water in a liquid mixture
B)partial pressure of water in a gaseous mixture
C)condensation of water
D)water dissolved in a liquid
E)water molecules
A)partial pressure of water in a liquid mixture
B)partial pressure of water in a gaseous mixture
C)condensation of water
D)water dissolved in a liquid
E)water molecules

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
76
A syringe contains 589 mL of CO at 325 K and 1.2 bar. A second syringe contains 473 mL of N2 at 298 K and 2.6 bar. What is the final pressure if the contents of these two syringes are injected into a 1.00 L container at STP?
A)0.59 bar
B)1.1 bar
C)1.7 bar
D)1.9 bar
E)3.8 bar
A)0.59 bar
B)1.1 bar
C)1.7 bar
D)1.9 bar
E)3.8 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
77
A mixture of He, Ne, and Ar has a pressure of 7.85 bar. If the Ne has a mole fraction of 0.47 and Ar has a mole fraction of 0.23, what is the pressure of He?
A)4.2 bar
B)3.7 bar
C)1.8 bar
D)5.5 bar
E)2.4 bar
A)4.2 bar
B)3.7 bar
C)1.8 bar
D)5.5 bar
E)2.4 bar

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
78
The mole fraction of oxygen in dry air near sea level is 0.20948. The concentration of oxygen is ________ molecules per litre, assuming an atmospheric pressure of 0.985 bar and a temperature of 29.5 °C.
A)6.23
B)0.00819
C)4.94 × 1021
D)3.75 × 1024
E)5.07 × 1022
A)6.23
B)0.00819
C)4.94 × 1021
D)3.75 × 1024
E)5.07 × 1022

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
79
When 14.0 g of zinc metal reacts with excess HCl, how many litres of H2 gas are produced at STP? Zn(s)+ 2HCl(aq)→ ZnCl2(aq)+ H2(g)
A)0.208 L
B)0.416 L
C)4.86 L
D)9.60 L
A)0.208 L
B)0.416 L
C)4.86 L
D)9.60 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
80
Determine the volume of O2 (at STP)formed when 50.0 g of KClO3 decomposes according to the following reaction. The molar mass for KClO3 is 122.55 g mol-1. 2KClO3(s)→ 2KCl(s)+ 3O2(g)
A)9.14 L
B)8.22 L
C)12.3 L
D)13.9 L
E)14.6 L
A)9.14 L
B)8.22 L
C)12.3 L
D)13.9 L
E)14.6 L

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck


