Deck 13: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/162
Play
Full screen (f)
Deck 13: Chemical Kinetics
1
Given the following balanced equation, determine the rate of reaction with respect to [NH3]. N2(g)+ 3H2(g)→ 2NH3(g)
A)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_8810_8b3b_c54f79bc91fe_TB6103_11.jpg)
B)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae21_8b3b_9b062961997f_TB6103_11.jpg)
C)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae22_8b3b_83da0ce9c1b4_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae23_8b3b_2fe6531f96da_TB6103_11.jpg)
D)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae24_8b3b_71ec7790f312_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae25_8b3b_f7531bb4546d_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_8810_8b3b_c54f79bc91fe_TB6103_11.jpg)
B)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae21_8b3b_9b062961997f_TB6103_11.jpg)
C)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae22_8b3b_83da0ce9c1b4_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae23_8b3b_2fe6531f96da_TB6103_11.jpg)
D)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae24_8b3b_71ec7790f312_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_ae25_8b3b_f7531bb4546d_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
Rate = + 
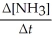

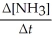
2
Given the following balanced equation, determine the rate of reaction with respect to [SO3]. 2SO2(g)+ O2(g)→ 2SO3(g)
A)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d8e_8b3b_0d18258a99aa_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d8f_8b3b_5333ec38a629_TB6103_11.jpg)
B)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d90_8b3b_13319c4f9f8b_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d91_8b3b_bb7bc0ed6260_TB6103_11.jpg)
C)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d92_8b3b_5b858bd1af59_TB6103_11.jpg)
D)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a3_8b3b_bb257b2d0855_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d8e_8b3b_0d18258a99aa_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d8f_8b3b_5333ec38a629_TB6103_11.jpg)
B)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d90_8b3b_13319c4f9f8b_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d91_8b3b_bb7bc0ed6260_TB6103_11.jpg)
C)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_9d92_8b3b_5b858bd1af59_TB6103_11.jpg)
D)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>3</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a3_8b3b_bb257b2d0855_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
Rate = + 
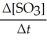

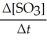
3
Given the following balanced equation, determine the rate of reaction with respect to [H2]. N2(g)+ 3H2(g)→ 2NH3(g)
A)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60f9_8b3b_276fa64a20e2_TB6103_11.jpg)
B)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60fa_8b3b_97ee5c9e5b03_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60fb_8b3b_237094d02d00_TB6103_11.jpg)
C)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880c_8b3b_c11e84e16066_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880d_8b3b_cd5ea16d9549_TB6103_11.jpg)
D)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880e_8b3b_33f1eaa7f5c5_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880f_8b3b_5be33807f01e_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60f9_8b3b_276fa64a20e2_TB6103_11.jpg)
B)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60fa_8b3b_97ee5c9e5b03_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60fb_8b3b_237094d02d00_TB6103_11.jpg)
C)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880c_8b3b_c11e84e16066_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880d_8b3b_cd5ea16d9549_TB6103_11.jpg)
D)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880e_8b3b_33f1eaa7f5c5_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)</strong> A)Rate = + B)Rate = - C)Rate = + D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_880f_8b3b_5be33807f01e_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
Rate = - 
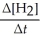

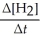
4
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the rate of formation of O2 is 6.94 × 10-1 mol L-1 s-1, what is the rate of the loss of O3? 2O3(g)→ 3O2(g)
A)0.463 mol L-1 s-1
B)1.04 mol L-1 s-1
C)2.08 mol L-1 s-1
D)0.231 mol L-1 s-1
E)4.16 mol L-1 s-1
A)0.463 mol L-1 s-1
B)1.04 mol L-1 s-1
C)2.08 mol L-1 s-1
D)0.231 mol L-1 s-1
E)4.16 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
5
Given the following balanced equation, determine the rate of reaction with respect to [SO2]. 2SO2(g)+ O2(g)→ 2SO3(g)
A)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_2852_8b3b_197f4ff658e7_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_2853_8b3b_31f779bfd46c_TB6103_11.jpg)
B)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f64_8b3b_8f26a66b1936_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f65_8b3b_19238718465f_TB6103_11.jpg)
C)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f66_8b3b_6bfb43a984e2_TB6103_11.jpg)
D)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f67_8b3b_8993c7833981_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_2852_8b3b_197f4ff658e7_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_2853_8b3b_31f779bfd46c_TB6103_11.jpg)
B)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f64_8b3b_8f26a66b1936_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f65_8b3b_19238718465f_TB6103_11.jpg)
C)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f66_8b3b_6bfb43a984e2_TB6103_11.jpg)
D)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [SO<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_4f67_8b3b_8993c7833981_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
6
Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. If the rate of Cl2 loss is 4.84 × 10-2 mol L-1 s-1, what is the rate of formation of NOCl? 2NO(g)+ Cl2(g)→ 2NOCl(g)
A)4.84 × 10-2 mol L-1 s-1
B)2.42 × 10-2 mol L-1 s-1
C)1.45 × 10-1 mol L-1 s-1
D)9.68 × 10-2 mol L-1 s-1
E)1.61 × 10-2 mol L-1 s-1
A)4.84 × 10-2 mol L-1 s-1
B)2.42 × 10-2 mol L-1 s-1
C)1.45 × 10-1 mol L-1 s-1
D)9.68 × 10-2 mol L-1 s-1
E)1.61 × 10-2 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
7
Given the following balanced equation, determine the rate of reaction with respect to [O2]. 2SO2(g)+ O2(g)→ 2SO3(g)
A)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_7678_8b3b_d1f23f557589_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_7679_8b3b_0750c924a599_TB6103_11.jpg)
B)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767a_8b3b_e1532b7179c1_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767b_8b3b_9fe4a3529369_TB6103_11.jpg)
C)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767c_8b3b_0555b518b285_TB6103_11.jpg)
D)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767d_8b3b_c7044aa69808_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_7678_8b3b_d1f23f557589_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_7679_8b3b_0750c924a599_TB6103_11.jpg)
B)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767a_8b3b_e1532b7179c1_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767b_8b3b_9fe4a3529369_TB6103_11.jpg)
C)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767c_8b3b_0555b518b285_TB6103_11.jpg)
D)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2SO<sub>3</sub>(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_767d_8b3b_c7044aa69808_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
8
Without performing complex calculations, determine the order of a reaction that has the following initial rates for the specified starting concentration: 
A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the methods used to monitor a reaction as it occurs in the reaction flask.
A)polarimeter
B)spectrometer
C)pressure measurement
D)none of the above
E)all of the above
A)polarimeter
B)spectrometer
C)pressure measurement
D)none of the above
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
10
Without performing complex calculations, determine the order of a reaction that has the following initial rates for the specified starting concentration: 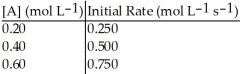
A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
11
Given the following balanced equation, determine the rate of reaction with respect to [SO3]. If the rate of SO2 loss is 1.19 × 10-3 mol L-1 s-1, what is the rate of formation of SO3? 2SO2(g)+ O2(g)→ 2SO3(g)
A)3.56 × 10-3 mol L-1 s-1
B)1.19 × 10-3 mol L-1 s-1
C)1.78 × 10-3 mol L-1 s-1
D)1.42 × 10-2 mol L-1 s-1
E)7.12 × 10-3 mol L-1 s-1
A)3.56 × 10-3 mol L-1 s-1
B)1.19 × 10-3 mol L-1 s-1
C)1.78 × 10-3 mol L-1 s-1
D)1.42 × 10-2 mol L-1 s-1
E)7.12 × 10-3 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
12
Given the following balanced equation, determine the rate of reaction with respect to [O2]. 2O3(g)→ 3O2(g)
A)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebbb_8b3b_c53f797851ee_TB6103_11.jpg)
B)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12cc_8b3b_0fac82c335e2_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12cd_8b3b_cb4c2bbbaa36_TB6103_11.jpg)
C)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12ce_8b3b_cb7cc1a52e49_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12cf_8b3b_9d41b62f8967_TB6103_11.jpg)
D)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12d0_8b3b_53bdd79271b7_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebbb_8b3b_c53f797851ee_TB6103_11.jpg)
B)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12cc_8b3b_0fac82c335e2_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12cd_8b3b_cb4c2bbbaa36_TB6103_11.jpg)
C)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12ce_8b3b_cb7cc1a52e49_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12cf_8b3b_9d41b62f8967_TB6103_11.jpg)
D)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>2</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_12d0_8b3b_53bdd79271b7_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
13
Write a balanced reaction for which the following rate relationships are true. Rate = - 
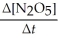 =
= 
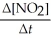 =
= 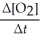
A)2N2O5 → 4NO2 + O2
B)4NO2 + O2 → 2N2O5
C)2N2O5 → NO2 + 4O2
D) NO2 + O2 →
NO2 + O2 →
 N2O5
N2O5
E) N2O5 →
N2O5 →
 NO2 + O2
NO2 + O2

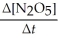 =
= 
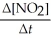 =
= 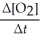
A)2N2O5 → 4NO2 + O2
B)4NO2 + O2 → 2N2O5
C)2N2O5 → NO2 + 4O2
D)
 NO2 + O2 →
NO2 + O2 →  N2O5
N2O5E)
 N2O5 →
N2O5 →  NO2 + O2
NO2 + O2
Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
14
Write a balanced reaction for which the following rate relationships are true. Rate = 
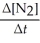 =
= 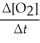 = -
= - 
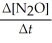
A) N2 + O2 →
N2 + O2 →
 N2O
N2O
B)2N2O → 2N2 + O2
C)N2O → N2 + 2O2
D) N2O →
N2O →  N2 + O2
N2 + O2
E)2N2 + O2 → 2N2O

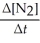 =
= 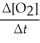 = -
= - 
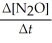
A)
 N2 + O2 →
N2 + O2 →  N2O
N2OB)2N2O → 2N2 + O2
C)N2O → N2 + 2O2
D)
 N2O →
N2O →  N2 + O2
N2 + O2E)2N2 + O2 → 2N2O

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
15
Given the following balanced equation, determine the rate of reaction with respect to [SO3]. If the rate of O2 loss is 3.56 × 10-3 mol L-1 s-1, what is the rate of formation of SO3? 2SO2(g)+ O2(g)→ 2SO3(g)
A)3.56 × 10-3 mol L-1 s-1
B)1.19 × 10-3 mol L-1 s-1
C)1.78 × 10-3 mol L-1 s-1
D)1.42 × 10-2 mol L-1 s-1
E)7.12 × 10-3 mol L-1 s-1
A)3.56 × 10-3 mol L-1 s-1
B)1.19 × 10-3 mol L-1 s-1
C)1.78 × 10-3 mol L-1 s-1
D)1.42 × 10-2 mol L-1 s-1
E)7.12 × 10-3 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
16
Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl2(g)→ 2NOCl(g)
A)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a4_8b3b_67b9866b6195_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a5_8b3b_3930aa4dbd4b_TB6103_11.jpg)
B)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a6_8b3b_f7e64a466ac8_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a7_8b3b_43a5090eba4a_TB6103_11.jpg)
C)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebb8_8b3b_15c487a981d3_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebb9_8b3b_f1e6b634765b_TB6103_11.jpg)
D)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebba_8b3b_a946686c36bc_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a4_8b3b_67b9866b6195_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a5_8b3b_3930aa4dbd4b_TB6103_11.jpg)
B)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a6_8b3b_f7e64a466ac8_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_c4a7_8b3b_43a5090eba4a_TB6103_11.jpg)
C)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebb8_8b3b_15c487a981d3_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebb9_8b3b_f1e6b634765b_TB6103_11.jpg)
D)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2NO(g)+ Cl<sub>2</sub>(g)→ 2NOCl(g)</strong> A)Rate = - B)Rate = + C)Rate = - D)Rate = - E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9665_ebba_8b3b_a946686c36bc_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
17
Without performing complex calculations, determine the order of a reaction that has the following initial rates for the specified starting concentration: 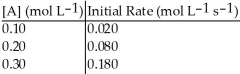
A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
18
Given the following balanced equation, determine the rate of reaction with respect to [O3]. 2O3(g)→ 3O2(g)
A)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e1_8b3b_3317a1e11f0e_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e2_8b3b_bba98969f0b4_TB6103_11.jpg)
B)Rate = -![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e3_8b3b_03600f33021d_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e4_8b3b_2b8f5b73c6ee_TB6103_11.jpg)
C)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e5_8b3b_4b6d96340221_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e6_8b3b_29d1471048fe_TB6103_11.jpg)
D)Rate = +![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60f7_8b3b_9b92cdfb7467_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60f8_8b3b_9d9632d0647d_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.
A)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e1_8b3b_3317a1e11f0e_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e2_8b3b_bba98969f0b4_TB6103_11.jpg)
B)Rate = -
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e3_8b3b_03600f33021d_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e4_8b3b_2b8f5b73c6ee_TB6103_11.jpg)
C)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e5_8b3b_4b6d96340221_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_39e6_8b3b_29d1471048fe_TB6103_11.jpg)
D)Rate = +
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60f7_8b3b_9b92cdfb7467_TB6103_11.jpg)
![<strong>Given the following balanced equation, determine the rate of reaction with respect to [O<sub>3</sub>]. 2O<sub>3</sub>(g)→ 3O<sub>2</sub>(g)</strong> A)Rate = - B)Rate = - C)Rate = + D)Rate = + E)It is not possible to determine the answer without more information.](https://storage.examlex.com/TB6103/11ea67b3_9666_60f8_8b3b_9d9632d0647d_TB6103_11.jpg)
E)It is not possible to determine the answer without more information.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
19
Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. If the rate of NO loss is 1.68 × 10-2 mol L-1 s-1, what is the rate of formation of NOCl? 2NO(g)+ Cl2(g)→ 2NOCl(g)
A)1.68 × 10-2 mol L-1 s-1
B)3.81 × 10-2 mol L-1 s-1
C)5.05 × 10-2 mol L-1 s-1
D)6.14 × 10-2 mol L-1 s-1
E)1.45 × 10-2 mol L-1 s-1
A)1.68 × 10-2 mol L-1 s-1
B)3.81 × 10-2 mol L-1 s-1
C)5.05 × 10-2 mol L-1 s-1
D)6.14 × 10-2 mol L-1 s-1
E)1.45 × 10-2 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
20
Given the following balanced equation, determine the rate of reaction with respect to [O3]. If the rate of floss of O3 is 3.91 × 10-1 mol L-1 s-1, what is the rate of the loss of O3? 2O3(g)→ 3O2(g)
A)2.61 mol L-1 s-1
B)0.937 mol L-1 s-1
C)0.261 mol L-1 s-1
D)0.587 mol L-1 s-1
E)0.817 mol L-1 s-1
A)2.61 mol L-1 s-1
B)0.937 mol L-1 s-1
C)0.261 mol L-1 s-1
D)0.587 mol L-1 s-1
E)0.817 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
21
Without performing complex calculations, determine the order of a reaction that has the following initial rates for the specified starting concentration: 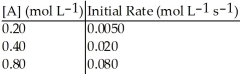
A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
22
What are the units of k in a first-order reaction?
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)M2 s-1
E)s-1
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)M2 s-1
E)s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
23
What is the overall order of the following reaction, given the rate law? NO(g)+ O3(g)→ NO2(g)+ O2(g)Rate = k[NO][O3]
A)1st order
B)2nd order
C)3rd order
D)1![<strong>What is the overall order of the following reaction, given the rate law? NO(g)+ O<sub>3</sub>(g)→ NO<sub>2</sub>(g)+ O<sub>2</sub>(g)Rate = k[NO][O<sub>3</sub>]</strong> A)1st order B)2nd order C)3rd order D)1 order E)0th order](https://storage.examlex.com/TB6103/11ea67b3_9668_34e3_8b3b_871bdf3a35c3_TB6103_11.jpg) order
order
E)0th order
A)1st order
B)2nd order
C)3rd order
D)1
![<strong>What is the overall order of the following reaction, given the rate law? NO(g)+ O<sub>3</sub>(g)→ NO<sub>2</sub>(g)+ O<sub>2</sub>(g)Rate = k[NO][O<sub>3</sub>]</strong> A)1st order B)2nd order C)3rd order D)1 order E)0th order](https://storage.examlex.com/TB6103/11ea67b3_9668_34e3_8b3b_871bdf3a35c3_TB6103_11.jpg) order
orderE)0th order

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is the characteristic of a second-order reaction having only one reactant.
A)The rate of the reaction is not proportional to the concentration of the reactant.
B)The rate of the reaction is proportional to the square of the concentration of the reactant.
C)The rate of the reaction is proportional to the square root of the concentration of the reactant.
D)The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E)The rate of the reaction is directly proportional to the concentration of the reactant.
A)The rate of the reaction is not proportional to the concentration of the reactant.
B)The rate of the reaction is proportional to the square of the concentration of the reactant.
C)The rate of the reaction is proportional to the square root of the concentration of the reactant.
D)The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E)The rate of the reaction is directly proportional to the concentration of the reactant.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is the characteristic of a first-order reaction having only one reactant?
A)The rate of the reaction is not proportional to the concentration of the reactant.
B)The rate of the reaction is proportional to the square of the concentration of the reactant.
C)The rate of the reaction is proportional to the square root of the concentration of the reactant.
D)The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E)The rate of the reaction is directly proportional to the concentration of the reactant.
A)The rate of the reaction is not proportional to the concentration of the reactant.
B)The rate of the reaction is proportional to the square of the concentration of the reactant.
C)The rate of the reaction is proportional to the square root of the concentration of the reactant.
D)The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E)The rate of the reaction is directly proportional to the concentration of the reactant.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
26
What are the units of k in a zero-order reaction?
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)M2 s-1
E)s-1
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)M2 s-1
E)s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
27
What are the units of k in the following rate law? Rate = k[X][Y]
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A)mol L<sup>-1</sup> s<sup>-1</sup> B)mol L<sup>-1</sup> s C)L mol<sup>-1 </sup>s<sup>-1</sup> D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8308_8b3b_63d7bf8c90e5_TB6103_11.jpg)
E)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A)mol L<sup>-1</sup> s<sup>-1</sup> B)mol L<sup>-1</sup> s C)L mol<sup>-1 </sup>s<sup>-1</sup> D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8309_8b3b_17e7b6ebb893_TB6103_11.jpg)
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A)mol L<sup>-1</sup> s<sup>-1</sup> B)mol L<sup>-1</sup> s C)L mol<sup>-1 </sup>s<sup>-1</sup> D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8308_8b3b_63d7bf8c90e5_TB6103_11.jpg)
E)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A)mol L<sup>-1</sup> s<sup>-1</sup> B)mol L<sup>-1</sup> s C)L mol<sup>-1 </sup>s<sup>-1</sup> D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8309_8b3b_17e7b6ebb893_TB6103_11.jpg)

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
28
What is the overall order of the following reaction, given the rate law? 2 X + 3 Y → 2 Z Rate = k[X]1[Y]2
A)3rd order
B)5th order
C)2nd order
D)1st order
E)0th order
A)3rd order
B)5th order
C)2nd order
D)1st order
E)0th order

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
29
What are the units of k in the following rate law? Rate = k[X]2
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)L mol-2 s-1
E)M2 s-1
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)L mol-2 s-1
E)M2 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
30
What is the overall order of the following reaction, given the rate law? 2NO(g)+ H2(g)→ N2(g)+ 2H2O(g)Rate = k[NO]2[H2]
A)1st order
B)2nd order
C)3rd order
D)4th order
E)0th order
A)1st order
B)2nd order
C)3rd order
D)4th order
E)0th order

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
31
What is the overall order of the following reaction, given the rate law? X + 2 Y → 4 Z Rate = k[X][Y]
A)3rd order
B)5th order
C)2nd order
D)1st order
E)0th order
A)3rd order
B)5th order
C)2nd order
D)1st order
E)0th order

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
32
Determine the missing initial rate for the following  order reaction given the following information:
order reaction given the following information: 
A)0.48 mol L-1 s-1
B)0.38 mol L-1 s-1
C)0.91 mol L-1 s-1
D)1.3 mol L-1 s-1
E)0.84 mol L-1 s-1
 order reaction given the following information:
order reaction given the following information: 
A)0.48 mol L-1 s-1
B)0.38 mol L-1 s-1
C)0.91 mol L-1 s-1
D)1.3 mol L-1 s-1
E)0.84 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
33
Determine the missing initial rate for the following  order reaction given the following information:
order reaction given the following information: 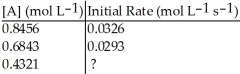
A)0.0352 mol L-1 s-1
B)0.0301 mol L-1 s-1
C)0.0215 mol L-1 s-1
D)0.0233 mol L-1 s-1
E)0.0280 mol L-1 s-1
 order reaction given the following information:
order reaction given the following information: 
A)0.0352 mol L-1 s-1
B)0.0301 mol L-1 s-1
C)0.0215 mol L-1 s-1
D)0.0233 mol L-1 s-1
E)0.0280 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
34
What are the units of k in the following rate law? Rate = k[X][Y]2
A)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_5bf4_8b3b_396c0ffeeeb3_TB6103_11.jpg)
B)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_5bf5_8b3b_c116dde6003d_TB6103_11.jpg)
C)M2 s
D)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8306_8b3b_29e662b4a81f_TB6103_11.jpg)
E)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8307_8b3b_69fb1541f527_TB6103_11.jpg)
A)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_5bf4_8b3b_396c0ffeeeb3_TB6103_11.jpg)
B)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_5bf5_8b3b_c116dde6003d_TB6103_11.jpg)
C)M2 s
D)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8306_8b3b_29e662b4a81f_TB6103_11.jpg)
E)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]<sup>2</sup></strong> A) B) C)M<sup>2</sup> s D) E)](https://storage.examlex.com/TB6103/11ea67b3_9668_8307_8b3b_69fb1541f527_TB6103_11.jpg)

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
35
What are the units of k in a second-order reaction?
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)M2 s-1
E)s-1
A)mol L-1 s-1
B)mol L-1 s
C)L mol-1 s-1
D)M2 s-1
E)s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
36
Without performing complex calculations, determine the order of a reaction that has the following initial rates for the specified starting concentration: 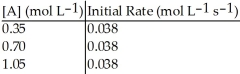
A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

A)zero order
B)first order
C)second order
D)third order
E)impossible to determine

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
37
What are the units of k in the following rate law? Rate = k[X][Y]1/2
A)mol L-1 s-1
B)L mol-1 s-1
C)L mol-1/2 s-1
D)L1/2 mol-1/2 s-1
E)L mol-1 s-1/2
A)mol L-1 s-1
B)L mol-1 s-1
C)L mol-1/2 s-1
D)L1/2 mol-1/2 s-1
E)L mol-1 s-1/2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
38
Given the following rate law, how does the rate of reaction change if the concentration of Y is doubled? Rate = k [X][Y]2
A)The rate of reaction will increase by a factor of 2.
B)The rate of reaction will increase by a factor of 4.
C)The rate of reaction will increase by a factor of 5.
D)The rate of reaction will decrease by a factor of 2.
E)The rate of reaction will remain unchanged.
A)The rate of reaction will increase by a factor of 2.
B)The rate of reaction will increase by a factor of 4.
C)The rate of reaction will increase by a factor of 5.
D)The rate of reaction will decrease by a factor of 2.
E)The rate of reaction will remain unchanged.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the characteristic of a zero-order reaction having only one reactant?
A)The rate of the reaction is not proportional to the concentration of the reactant.
B)The rate of the reaction is proportional to the square of the concentration of the reactant.
C)The rate of the reaction is proportional to the square root of the concentration of the reactant.
D)The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E)The rate of the reaction is directly proportional to the concentration of the reactant.
A)The rate of the reaction is not proportional to the concentration of the reactant.
B)The rate of the reaction is proportional to the square of the concentration of the reactant.
C)The rate of the reaction is proportional to the square root of the concentration of the reactant.
D)The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E)The rate of the reaction is directly proportional to the concentration of the reactant.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
40
Determine the missing initial rate for a reaction with an order of  given the following information:
given the following information: 
A)6.0 × 10-3 mol L-1 s-1
B)2.8 × 10-2 mol L-1 s-1
C)7.3 × 10-3 mol L-1 s-1
D)9.74 × 10-3 mol L-1 s-1
E)1.0 × 10-2 mol L-1 s-1
 given the following information:
given the following information: 
A)6.0 × 10-3 mol L-1 s-1
B)2.8 × 10-2 mol L-1 s-1
C)7.3 × 10-3 mol L-1 s-1
D)9.74 × 10-3 mol L-1 s-1
E)1.0 × 10-2 mol L-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
41
Given the following rate law, how does the rate of reaction change if the concentration of X is doubled? Rate = k [X]2[Y]3
A)The rate of reaction will increase by a factor of 9.
B)The rate of reaction will increase by a factor of 2.
C)The rate of reaction will increase by a factor of 8.
D)The rate of reaction will increase by a factor of 4.
E)The rate of reaction will remain unchanged.
A)The rate of reaction will increase by a factor of 9.
B)The rate of reaction will increase by a factor of 2.
C)The rate of reaction will increase by a factor of 8.
D)The rate of reaction will increase by a factor of 4.
E)The rate of reaction will remain unchanged.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
42
What data should be plotted to show that experimental concentration data fit a second-order reaction?
A)ln[reactant] vs. time
B)[reactant] vs. time
C)ln(k)vs.![<strong>What data should be plotted to show that experimental concentration data fit a second-order reaction?</strong> A)ln[reactant] vs. time B)[reactant] vs. time C)ln(k)vs. D) <sub> </sub>vs. time E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_bb9e_8b3b_a59dc59c4059_TB6103_11.jpg)
D)![<strong>What data should be plotted to show that experimental concentration data fit a second-order reaction?</strong> A)ln[reactant] vs. time B)[reactant] vs. time C)ln(k)vs. D) <sub> </sub>vs. time E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_bb9f_8b3b_b97e16f0b408_TB6103_11.jpg) vs. time
vs. time
E)ln(k)vs. Ea
A)ln[reactant] vs. time
B)[reactant] vs. time
C)ln(k)vs.
![<strong>What data should be plotted to show that experimental concentration data fit a second-order reaction?</strong> A)ln[reactant] vs. time B)[reactant] vs. time C)ln(k)vs. D) <sub> </sub>vs. time E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_bb9e_8b3b_a59dc59c4059_TB6103_11.jpg)
D)
![<strong>What data should be plotted to show that experimental concentration data fit a second-order reaction?</strong> A)ln[reactant] vs. time B)[reactant] vs. time C)ln(k)vs. D) <sub> </sub>vs. time E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_bb9f_8b3b_b97e16f0b408_TB6103_11.jpg) vs. time
vs. timeE)ln(k)vs. Ea

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
43
Given the following rate law, how does the rate of reaction change if the concentration of Y is doubled? Rate = k [X]2[Y]3
A)The rate of reaction will increase by a factor of 9.
B)The rate of reaction will increase by a factor of 2.
C)The rate of reaction will increase by a factor of 8.
D)The rate of reaction will increase by a factor of 4.
E)The rate of reaction will remain unchanged.
A)The rate of reaction will increase by a factor of 9.
B)The rate of reaction will increase by a factor of 2.
C)The rate of reaction will increase by a factor of 8.
D)The rate of reaction will increase by a factor of 4.
E)The rate of reaction will remain unchanged.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
44
How many half-lives are required for the concentration of reactant to decrease to 25% of its original value?
A)1
B)3
C)1.5
D)2.5
E)2
A)1
B)3
C)1.5
D)2.5
E)2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the rate law and the value of k for the following reaction using the data provided: CO(g)+ Cl2(g)→ COCl2(g)[CO]i (M)[Cl2]i (M)Initial Rate (M1 s-1)
0.25 0.40 0.696
0.25 0.80 1.97
0.50 0.80 3.94
A)Rate = 11 M-3/2 s-1 [CO][Cl2]3/2
B)Rate = 36 M-1.8 s-1 [CO][Cl2]2.8
C)Rate = 17 M-2 s-1 [CO][Cl2]2
D)Rate = 4.4 M-1/2 s-1 [CO][Cl2]1/2
E)Rate = 18 M-3/2 s-1 [CO]2[Cl2]1/2
0.25 0.40 0.696
0.25 0.80 1.97
0.50 0.80 3.94
A)Rate = 11 M-3/2 s-1 [CO][Cl2]3/2
B)Rate = 36 M-1.8 s-1 [CO][Cl2]2.8
C)Rate = 17 M-2 s-1 [CO][Cl2]2
D)Rate = 4.4 M-1/2 s-1 [CO][Cl2]1/2
E)Rate = 18 M-3/2 s-1 [CO]2[Cl2]1/2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following represents the equation for a second-order half-life?
A)t 1/2 =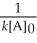
B)t 1/2 =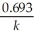
C)t 1/2 =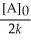
D)t 1/2 =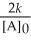
E)t 1/2 =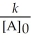
A)t 1/2 =
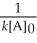
B)t 1/2 =
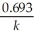
C)t 1/2 =
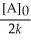
D)t 1/2 =
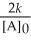
E)t 1/2 =
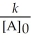

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
47
Determine the rate law and the value of k for the following reaction using the data provided: NO2(g)+ O3(g)→ NO3(g)+ O2(g)[NO2]i (M)[O3]i (M)Initial Rate (M-1 s-1)
0.10 0.33 1.42
0.10 0.66 2.84
0.25 0.66 7.10
A)Rate = 1360 M-2.5 s-1[NO2]2.5[O3]
B)Rate = 227 M-2.5 s-1[NO2][O3]2.5
C)Rate = 43 M-1 s-1[NO2][O3]
D)Rate = 430 M-2 s-1[NO2]2[O3]
E)Rate = 130 M-2 s-1[NO2][O3]2
0.10 0.33 1.42
0.10 0.66 2.84
0.25 0.66 7.10
A)Rate = 1360 M-2.5 s-1[NO2]2.5[O3]
B)Rate = 227 M-2.5 s-1[NO2][O3]2.5
C)Rate = 43 M-1 s-1[NO2][O3]
D)Rate = 430 M-2 s-1[NO2]2[O3]
E)Rate = 130 M-2 s-1[NO2][O3]2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
48
Given the following rate law, how does the rate of reaction change if the concentration of Y is doubled? Rate = k [X][Y]
A)The rate of reaction will increase by a factor of 2.
B)The rate of reaction will increase by a factor of 4.
C)The rate of reaction will increase by a factor of 5.
D)The rate of reaction will decrease by a factor of 2.
E)The rate of reaction will remain unchanged.
A)The rate of reaction will increase by a factor of 2.
B)The rate of reaction will increase by a factor of 4.
C)The rate of reaction will increase by a factor of 5.
D)The rate of reaction will decrease by a factor of 2.
E)The rate of reaction will remain unchanged.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the rate law and the value of k for the following reaction using the data provided: 2NO(g)+ O2(g)→ 2NO2(g)[NO]i (M)[O2]i (M)Initial Rate (M-1 s-1)
0.030 0.0055 8.55 × 10-3
0.030 0.0110 1.71 × 10-2
0.060 0.0055 3.42 × 10-2
A)Rate = 57 M-1 s-1[NO][O2]
B)Rate = 3.8 M-1/2 s-1[NO][O2]1/2
C)Rate = 3.1 × 105 M-3 s-1[NO]2[O2]2
D)Rate = 1.7 × 103 M-2 s-1[NO]2[O2]
E)Rate = 9.4 × 103 M-2 s-1[NO][O2]2
0.030 0.0055 8.55 × 10-3
0.030 0.0110 1.71 × 10-2
0.060 0.0055 3.42 × 10-2
A)Rate = 57 M-1 s-1[NO][O2]
B)Rate = 3.8 M-1/2 s-1[NO][O2]1/2
C)Rate = 3.1 × 105 M-3 s-1[NO]2[O2]2
D)Rate = 1.7 × 103 M-2 s-1[NO]2[O2]
E)Rate = 9.4 × 103 M-2 s-1[NO][O2]2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the rate law and the value of k for the following reaction using the data provided: S2O82⁻(aq)+ 3 I⁻(aq)→ 2 SO42⁻(g)+ I3⁻(aq)[S2O82⁻]i (M)[I⁻]i (M)Initial Rate (M-1 s-1)
0.30 0.42 4.54
0.44 0.42 6.65
0.44 0.21 3.33
A)Rate = 120 M-2 s-1 [S2O82⁻]2[I⁻]
B)Rate = 36 M-1 s-1 [S2O82⁻][I⁻]
C)Rate = 86 M-2 s-1 [S2O82⁻][I⁻]2
D)Rate = 195 M-3 s-1 [S2O82⁻]2[I⁻]2
E)Rate = 23 M-1/2 s-1 [S2O82⁻][I⁻]1/2
0.30 0.42 4.54
0.44 0.42 6.65
0.44 0.21 3.33
A)Rate = 120 M-2 s-1 [S2O82⁻]2[I⁻]
B)Rate = 36 M-1 s-1 [S2O82⁻][I⁻]
C)Rate = 86 M-2 s-1 [S2O82⁻][I⁻]2
D)Rate = 195 M-3 s-1 [S2O82⁻]2[I⁻]2
E)Rate = 23 M-1/2 s-1 [S2O82⁻][I⁻]1/2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the rate law and the value of k for the following reaction using the data provided: 2N2O5(g)→ 4NO2(g)+ O2(g)[N2O5]i (M)Initial Rate (M-1 s-1)
0.093 4.84 × 10-4
0.186 9.67 × 10-4
0.279 1.45 × 10-3
A)Rate = 5.6 × 10-2 M-1 s-1[N2O5]2
B)Rate = 6.0 × 10-1 M-2 s-1[N2O5]3
C)Rate = 1.6 × 10-3 M1/2 s-1[N2O5]1/2
D)Rate = 1.7 × 10-2 M-1/2 s-1[N2O5]3/2
E)Rate = 5.2 × 10⁻3 s-1[N2O5]
0.093 4.84 × 10-4
0.186 9.67 × 10-4
0.279 1.45 × 10-3
A)Rate = 5.6 × 10-2 M-1 s-1[N2O5]2
B)Rate = 6.0 × 10-1 M-2 s-1[N2O5]3
C)Rate = 1.6 × 10-3 M1/2 s-1[N2O5]1/2
D)Rate = 1.7 × 10-2 M-1/2 s-1[N2O5]3/2
E)Rate = 5.2 × 10⁻3 s-1[N2O5]

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following represents the integrated rate law for a first-order reaction?
A)![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4a_8b3b_d1d95f31e5de_TB6103_11.jpg) = -kt
= -kt
B)![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4b_8b3b_9b190670744e_TB6103_11.jpg) -
- ![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4c_8b3b_85ac407328eb_TB6103_11.jpg) = kt
= kt
C)[A]t - [A]0 = -kt
D)k = Ae(-Ea/RT)
E)![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4d_8b3b_07394699accb_TB6103_11.jpg) =
= ![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4e_8b3b_5bb7f10692c4_TB6103_11.jpg)
![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4f_8b3b_594e9b365b27_TB6103_11.jpg) + lnA
+ lnA
A)
![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4a_8b3b_d1d95f31e5de_TB6103_11.jpg) = -kt
= -ktB)
![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4b_8b3b_9b190670744e_TB6103_11.jpg) -
- ![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4c_8b3b_85ac407328eb_TB6103_11.jpg) = kt
= ktC)[A]t - [A]0 = -kt
D)k = Ae(-Ea/RT)
E)
![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4d_8b3b_07394699accb_TB6103_11.jpg) =
= ![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4e_8b3b_5bb7f10692c4_TB6103_11.jpg)
![<strong>Which of the following represents the integrated rate law for a first-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_1f4f_8b3b_594e9b365b27_TB6103_11.jpg) + lnA
+ lnA
Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following represents the integrated rate law for a zeroth-order reaction?
A)![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d76_8b3b_0fe87caedb5c_TB6103_11.jpg) = -kt
= -kt
B)![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d77_8b3b_cfa31e0ed4d8_TB6103_11.jpg) -
- ![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d78_8b3b_ff590d502335_TB6103_11.jpg) = kt
= kt
C)[A]t - [A]0 = -kt
D)k = Ae(-Ea/RT)
E)![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_9489_8b3b_d7b9ad279080_TB6103_11.jpg) =
= ![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_948a_8b3b_d329f7989d00_TB6103_11.jpg)
![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_948b_8b3b_056dfe3ce15d_TB6103_11.jpg) + lnA
+ lnA
A)
![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d76_8b3b_0fe87caedb5c_TB6103_11.jpg) = -kt
= -ktB)
![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d77_8b3b_cfa31e0ed4d8_TB6103_11.jpg) -
- ![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d78_8b3b_ff590d502335_TB6103_11.jpg) = kt
= ktC)[A]t - [A]0 = -kt
D)k = Ae(-Ea/RT)
E)
![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_9489_8b3b_d7b9ad279080_TB6103_11.jpg) =
= ![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_948a_8b3b_d329f7989d00_TB6103_11.jpg)
![<strong>Which of the following represents the integrated rate law for a zeroth-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_948b_8b3b_056dfe3ce15d_TB6103_11.jpg) + lnA
+ lnA
Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following represents the integrated rate law for a second-order reaction?
A)![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4660_8b3b_4d26ff9f38de_TB6103_11.jpg) = -kt
= -kt
B)![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4661_8b3b_49398cc5c798_TB6103_11.jpg) -
- ![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4662_8b3b_b5d5daa0a5ec_TB6103_11.jpg) = kt
= kt
C)[A]t - [A]0 = -kt
D)k = Ae(-Ea/RT)
E)![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4663_8b3b_4513202d27fb_TB6103_11.jpg) =
= ![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d74_8b3b_f1cc71e083a3_TB6103_11.jpg)
![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d75_8b3b_c7881c4e4111_TB6103_11.jpg) + lnA
+ lnA
A)
![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4660_8b3b_4d26ff9f38de_TB6103_11.jpg) = -kt
= -ktB)
![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4661_8b3b_49398cc5c798_TB6103_11.jpg) -
- ![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4662_8b3b_b5d5daa0a5ec_TB6103_11.jpg) = kt
= ktC)[A]t - [A]0 = -kt
D)k = Ae(-Ea/RT)
E)
![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_4663_8b3b_4513202d27fb_TB6103_11.jpg) =
= ![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d74_8b3b_f1cc71e083a3_TB6103_11.jpg)
![<strong>Which of the following represents the integrated rate law for a second-order reaction?</strong> A) = -kt B) - = kt C)[A]<sub>t</sub> - [A]<sub>0</sub> = -kt D)k = Ae<sup>(-</sup><sup>E</sup><sub>a</sub>/RT) E) = + lnA](https://storage.examlex.com/TB6103/11ea67b3_9669_6d75_8b3b_c7881c4e4111_TB6103_11.jpg) + lnA
+ lnA
Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
55
Given the following rate law, how does the rate of reaction change if the concentration of X is doubled? Rate = k [X][Y]2
A)The rate of reaction will increase by a factor of 2.
B)The rate of reaction will increase by a factor of 4.
C)The rate of reaction will increase by a factor of 5.
D)The rate of reaction will decrease by a factor of 2.
E)The rate of reaction will remain unchanged.
A)The rate of reaction will increase by a factor of 2.
B)The rate of reaction will increase by a factor of 4.
C)The rate of reaction will increase by a factor of 5.
D)The rate of reaction will decrease by a factor of 2.
E)The rate of reaction will remain unchanged.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
56
What data should be plotted to show that experimental concentration data fit a first-order reaction?
A)![<strong>What data should be plotted to show that experimental concentration data fit a first-order reaction?</strong> A) <sub> </sub>vs. time B)[reactant] vs. time C)ln[reactant] vs. time D)ln(k)vs. E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_948c_8b3b_bf0ae2695402_TB6103_11.jpg) vs. time
vs. time
B)[reactant] vs. time
C)ln[reactant] vs. time
D)ln(k)vs.![<strong>What data should be plotted to show that experimental concentration data fit a first-order reaction?</strong> A) <sub> </sub>vs. time B)[reactant] vs. time C)ln[reactant] vs. time D)ln(k)vs. E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_948d_8b3b_99a3ee1bf536_TB6103_11.jpg)
E)ln(k)vs. Ea
A)
![<strong>What data should be plotted to show that experimental concentration data fit a first-order reaction?</strong> A) <sub> </sub>vs. time B)[reactant] vs. time C)ln[reactant] vs. time D)ln(k)vs. E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_948c_8b3b_bf0ae2695402_TB6103_11.jpg) vs. time
vs. timeB)[reactant] vs. time
C)ln[reactant] vs. time
D)ln(k)vs.
![<strong>What data should be plotted to show that experimental concentration data fit a first-order reaction?</strong> A) <sub> </sub>vs. time B)[reactant] vs. time C)ln[reactant] vs. time D)ln(k)vs. E)ln(k)vs. E<sub>a</sub>](https://storage.examlex.com/TB6103/11ea67b3_9669_948d_8b3b_99a3ee1bf536_TB6103_11.jpg)
E)ln(k)vs. Ea

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
57
What data should be plotted to show that experimental concentration data fit a zeroth-order reaction?
A)ln[reactant] vs. time
B)![<strong>What data should be plotted to show that experimental concentration data fit a zeroth-order reaction?</strong> A)ln[reactant] vs. time B) <sub> </sub>vs. time C)ln(k)vs. D)ln(k)vs. E<sub>a</sub> E)[reactant] vs. time](https://storage.examlex.com/TB6103/11ea67b3_9669_bba0_8b3b_3be291ebf57d_TB6103_11.jpg) vs. time
vs. time
C)ln(k)vs.![<strong>What data should be plotted to show that experimental concentration data fit a zeroth-order reaction?</strong> A)ln[reactant] vs. time B) <sub> </sub>vs. time C)ln(k)vs. D)ln(k)vs. E<sub>a</sub> E)[reactant] vs. time](https://storage.examlex.com/TB6103/11ea67b3_9669_bba1_8b3b_195628321279_TB6103_11.jpg)
D)ln(k)vs. Ea
E)[reactant] vs. time
A)ln[reactant] vs. time
B)
![<strong>What data should be plotted to show that experimental concentration data fit a zeroth-order reaction?</strong> A)ln[reactant] vs. time B) <sub> </sub>vs. time C)ln(k)vs. D)ln(k)vs. E<sub>a</sub> E)[reactant] vs. time](https://storage.examlex.com/TB6103/11ea67b3_9669_bba0_8b3b_3be291ebf57d_TB6103_11.jpg) vs. time
vs. timeC)ln(k)vs.
![<strong>What data should be plotted to show that experimental concentration data fit a zeroth-order reaction?</strong> A)ln[reactant] vs. time B) <sub> </sub>vs. time C)ln(k)vs. D)ln(k)vs. E<sub>a</sub> E)[reactant] vs. time](https://storage.examlex.com/TB6103/11ea67b3_9669_bba1_8b3b_195628321279_TB6103_11.jpg)
D)ln(k)vs. Ea
E)[reactant] vs. time

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following represents the equation for a zero-order half-life?
A)t 1/2 =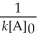
B)t 1/2 =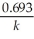
C)t 1/2 =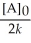
D)t 1/2 =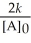
E)t 1/2 =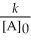
A)t 1/2 =
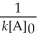
B)t 1/2 =
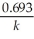
C)t 1/2 =
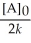
D)t 1/2 =
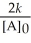
E)t 1/2 =
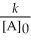

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements is FALSE?
A)The average rate of a reaction decreases during a reaction.
B)It is not possible to determine the rate of a reaction from its balanced equation.
C)The rate of zero-order reactions is not dependent on concentration.
D)The half-life of a first-order reaction is dependent on the initial concentration of reactant.
E)None of the above statements is false.
A)The average rate of a reaction decreases during a reaction.
B)It is not possible to determine the rate of a reaction from its balanced equation.
C)The rate of zero-order reactions is not dependent on concentration.
D)The half-life of a first-order reaction is dependent on the initial concentration of reactant.
E)None of the above statements is false.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following represents the equation for a first-order half-life?
A)t 1/2 =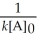
B)t 1/2 =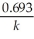
C)t 1/2 =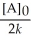
D)t 1/2 =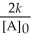
E)t 1/2 =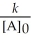
A)t 1/2 =
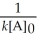
B)t 1/2 =
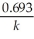
C)t 1/2 =
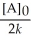
D)t 1/2 =
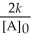
E)t 1/2 =
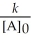

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
61
The first-order decomposition of N2O at 1000 K has a rate constant of 0.76 s-1. If the initial concentration of N2O is 10.9 mol L-1, what is the concentration of N2O after 9.6 s?
A)7.4 × 10-3 mol L-1
B)1.0 × 10-3 mol L-1
C)1.4 × 10-3 mol L-1
D)3.6 × 10-3 mol L-1
E)8.7 × 10-3 mol L-1
A)7.4 × 10-3 mol L-1
B)1.0 × 10-3 mol L-1
C)1.4 × 10-3 mol L-1
D)3.6 × 10-3 mol L-1
E)8.7 × 10-3 mol L-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
62
Derive an expression for a "1/3-life" for a first-order reaction.
A)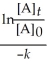
B)
C)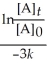
D)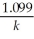
E)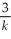
A)
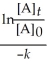
B)
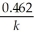
C)
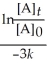
D)
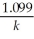
E)
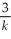

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
63
If the concentration of a reactant is 6.25%, how many half-lives has it gone through?
A)7
B)6
C)3
D)4
E)5
A)7
B)6
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
64
The rate constant for a second-order reaction is 0.54 L mol-1 s-1. What is the half-life of this reaction if the initial concentration is 0.27 mol L-1?
A)2.0 s
B)5.0 s
C)0.25 s
D)6.9 s
E)1.3 s
A)2.0 s
B)5.0 s
C)0.25 s
D)6.9 s
E)1.3 s

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
65
For a reaction, what generally happens if the temperature is increased?
A)a decrease in k occurs, which results in a faster rate
B)a decrease in k occurs, which results in a slower rate
C)an increase in k occurs, which results in a faster rate
D)an increase in k occurs, which results in a slower rate
E)there is no change to either k or the rate
A)a decrease in k occurs, which results in a faster rate
B)a decrease in k occurs, which results in a slower rate
C)an increase in k occurs, which results in a faster rate
D)an increase in k occurs, which results in a slower rate
E)there is no change to either k or the rate

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
66
The first-order decay of radon has a half-life of 3.823 days. How many grams of radon remain after 7.22 days if the sample initially weighs 250.0 grams?
A)4.21 g
B)183 g
C)54.8 g
D)76.3 g
E)67.5 g
A)4.21 g
B)183 g
C)54.8 g
D)76.3 g
E)67.5 g

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
67
The first-order decay of radon has a half-life of 3.823 days. How many grams of radon decomposes after 5.55 days if the sample initially weighs 100.0 grams?
A)83.4 g
B)16.6 g
C)50.0 g
D)36.6 g
E)63.4 g
A)83.4 g
B)16.6 g
C)50.0 g
D)36.6 g
E)63.4 g

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
68
The rate constant for a zero-order reaction is 0.54 L mol-1 s-1. What is the half-life of this reaction if the initial concentration is 0.27 mol L-1?
A)2.0 s
B)5.0 s
C)0.25 s
D)6.9 s
E)1.3 s
A)2.0 s
B)5.0 s
C)0.25 s
D)6.9 s
E)1.3 s

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
69
The half-life for the second-order decomposition of HI is 15.4 s when the initial concentration of HI is 0.67 mol L-1. What is the rate constant for this reaction?
A)1.0 × 10-2 L mol-1 s-1
B)4.5 × 10-2 L mol-1 s-1
C)9.7 × 10-2 L mol-1 s-1
D)2.2 × 10-2 L mol-1 s-1
E)3.8 × 10-2 L mol-1 s-1
A)1.0 × 10-2 L mol-1 s-1
B)4.5 × 10-2 L mol-1 s-1
C)9.7 × 10-2 L mol-1 s-1
D)2.2 × 10-2 L mol-1 s-1
E)3.8 × 10-2 L mol-1 s-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
70
The second-order decomposition of HI has a rate constant of 1.80 × 10-3 L mol-1 s-1. How much HI remains after 27.3 s if the initial concentration of HI is 4.78 mol L-1?
A)4.55 mol L-1
B)0.258 mol L-1
C)3.87 mol L-1
D)2.20 mol L-1
E)2.39 mol L-1
A)4.55 mol L-1
B)0.258 mol L-1
C)3.87 mol L-1
D)2.20 mol L-1
E)2.39 mol L-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
71
The first-order decomposition of cyclopropane has a rate constant of 6.7 × 10-4 s-1. If the initial concentration of cyclopropane is 1.33 mol L-1, what is the concentration of cyclopropane after 644 s?
A)0.43 mol L-1
B)0.15 mol L-1
C)0.94 mol L-1
D)0.86 mol L-1
E)0.67 mol L-1
A)0.43 mol L-1
B)0.15 mol L-1
C)0.94 mol L-1
D)0.86 mol L-1
E)0.67 mol L-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
72
The first-order decomposition of N2O5 at 328 K has a rate constant of 1.70 × 10-3 s-1. If the initial concentration of N2O5 is 2.88 mol L-1, what is the concentration of N2O5 after 12.5 minutes?
A)0.124 mol L-1
B)0.805 mol L-1
C)2.82 mol L-1
D)0.355 mol L-1
E)0.174 mol L-1
A)0.124 mol L-1
B)0.805 mol L-1
C)2.82 mol L-1
D)0.355 mol L-1
E)0.174 mol L-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
73
How many half-lives are required for the concentration of reactant to decrease to 12.5% of its original value?
A)3
B)1
C)1.75
D)2.75
E)2
A)3
B)1
C)1.75
D)2.75
E)2

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following reactions would you predict to have the smallest orientation factor?
A)X2 + Y2 → 2XY
B)NOCl2 + NO → 2 NOCl
C)N2 + O2 → 2NO
D)N + O2 → NO2
E)All of these reactions should have nearly identical orientation factors.
A)X2 + Y2 → 2XY
B)NOCl2 + NO → 2 NOCl
C)N2 + O2 → 2NO
D)N + O2 → NO2
E)All of these reactions should have nearly identical orientation factors.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
75
The half-life for the decay of radium is 1620 years. What is the rate constant for this first-order process?
A)4.28 × 10-4 yr-1
B)1.12 × 10-4 yr-1
C)2.33 × 10-4 yr-1
D)8.91 × 10-4 yr-1
E)6.17 × 10-4 yr-1
A)4.28 × 10-4 yr-1
B)1.12 × 10-4 yr-1
C)2.33 × 10-4 yr-1
D)8.91 × 10-4 yr-1
E)6.17 × 10-4 yr-1

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
76
Derive an expression for a "1/4-life" for a first-order reaction.
A)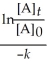
B)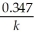
C)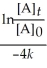
D)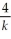
E)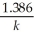
A)
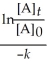
B)
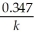
C)
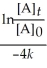
D)
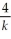
E)
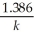

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
77
The second-order decomposition of NO2 has a rate constant of 0.255 L mol-1 s-1. How much NO2 decomposes in 4.00 s if the initial concentration of NO2 (1.00 L volume)is 1.33 mol L-1?
A)1.8 mol
B)0.85 mol
C)0.48 mol
D)0.77 mol
E)0.56 mol
A)1.8 mol
B)0.85 mol
C)0.48 mol
D)0.77 mol
E)0.56 mol

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
78
How many half-lives are required for the concentration of reactant to decrease to 1.56% of its original value?
A)6
B)5
C)7
D)6.5
E)7.5
A)6
B)5
C)7
D)6.5
E)7.5

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
79
The rate constant for the first-order decomposition of N2O is 3.40 s-1. What is the half-life of the decomposition?
A)0.491 s
B)0.204 s
C)0.236 s
D)0.424 s
E)0.294 s
A)0.491 s
B)0.204 s
C)0.236 s
D)0.424 s
E)0.294 s

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
80
The rate constant for a first-order reaction is 0.54 L mol-1 s-1. What is the half-life of this reaction if the initial concentration is 0.27 mol L-1?
A)2.0 s
B)5.0 s
C)0.25 s
D)6.9 s
E)1.3 s
A)2.0 s
B)5.0 s
C)0.25 s
D)6.9 s
E)1.3 s

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck


