Deck 26: Structures of Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 26: Structures of Organic Compounds
1
A conformation is a set of all possible optical isomers of an organic compound of a given molecular formula.
False
2
Which of the following classes of organic compounds and their general structural formulas have been correctly matched?
I.ester:
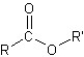
II.aldehyde:
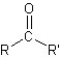
III.ketone:
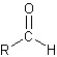
IV.carboxylic acid:
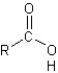
V.amide:
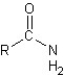
A)I,II,and V
B)II,III,and V
C)III,IV,and V
D)I,IV,and V
E)II,IV,and V
I.ester:

II.aldehyde:

III.ketone:

IV.carboxylic acid:

V.amide:

A)I,II,and V
B)II,III,and V
C)III,IV,and V
D)I,IV,and V
E)II,IV,and V
I,IV,and V
3
Replacing the acidic hydrogen of a carboxylic acid with an organic group produces an amide.
False
4
Cis-trans isomerism is a type of isomerism generally known as stereoisomerism.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
How many primary,secondary,tertiary and quaternary carbon atoms are there in the structure of 2,4,6-trimetylheptane?
A)5 primary,2 secondary,2 tertiary and 1 quaternary carbon atoms
B)5 primary,3 secondary,2 tertiary and 0 quaternary carbon atoms
C)4 primary,4 secondary,2 tertiary and 0 quaternary carbon atoms
D)4 primary,2 secondary,2 tertiary and 2 quaternary carbon atoms
E)5 primary,2 secondary,3 tertiary and 0 quaternary carbon atoms
A)5 primary,2 secondary,2 tertiary and 1 quaternary carbon atoms
B)5 primary,3 secondary,2 tertiary and 0 quaternary carbon atoms
C)4 primary,4 secondary,2 tertiary and 0 quaternary carbon atoms
D)4 primary,2 secondary,2 tertiary and 2 quaternary carbon atoms
E)5 primary,2 secondary,3 tertiary and 0 quaternary carbon atoms

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the INCORRECT statement.
A)Hydrocarbons are compounds of carbon and hydrogen.
B)Skeletal isomerism is also called chain isomerism.
C)Isomers have the same chemical formula but differ with respect to the arrangement of atoms.
D)Saturated hydrocarbons have only single bonds connecting each atom.
E)Organic compounds can be obtained only from living tissue.
A)Hydrocarbons are compounds of carbon and hydrogen.
B)Skeletal isomerism is also called chain isomerism.
C)Isomers have the same chemical formula but differ with respect to the arrangement of atoms.
D)Saturated hydrocarbons have only single bonds connecting each atom.
E)Organic compounds can be obtained only from living tissue.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Different forms of isomerism in organic compounds can be summarized as follows:
"Isomers can be constitutional or stereoisomers.Constitutional isomers are further divided in enantiomers and diastereomers."
"Isomers can be constitutional or stereoisomers.Constitutional isomers are further divided in enantiomers and diastereomers."

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
In alkene nomenclature "trans" stands for "on the same side" while "cis" means "across."

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
Choose the INCORRECT statement.
A)Latin prefixes provide information on the numbers of C atoms in a chain.
B)An alkane group used as a side chain group is called an alkyl group.
C)Di,tri,and tetra can be used to designate the number of side chain groups.
D)Positional isomers differ in the position on the main chain of the side chain group.
E)Functional groups are distinctive groupings of one or more atoms.
A)Latin prefixes provide information on the numbers of C atoms in a chain.
B)An alkane group used as a side chain group is called an alkyl group.
C)Di,tri,and tetra can be used to designate the number of side chain groups.
D)Positional isomers differ in the position on the main chain of the side chain group.
E)Functional groups are distinctive groupings of one or more atoms.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Which compound is the lowest boiling?
A)methane
B)ethene
C)ethane
D)cyclopropane
E)ethyne
A)methane
B)ethene
C)ethane
D)cyclopropane
E)ethyne

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Two structural features shared among all aromatic organic compounds are:
1)They all are flat,cyclic or linear molecules
2)They have a conjugated bonding system.
1)They all are flat,cyclic or linear molecules
2)They have a conjugated bonding system.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
When determining the priority of substituents is "A substituent atom of higher atomic number has higher priority over a substituent atom with lower atomic number."

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
The IUPAC name of tert-butyl group is:
A)1,1-dimethylethyl
B)1,1-dimethylethane
C)1,2-dimethylethyl
D)1-methylpropyl
E)2-methylpropyl
A)1,1-dimethylethyl
B)1,1-dimethylethane
C)1,2-dimethylethyl
D)1-methylpropyl
E)2-methylpropyl

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Constitutional isomers have different bond connectivities,and as a consequence they have different skeletal structures.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
In the rings of heterocyclic compounds we can find one or more atoms that are not carbon atoms.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
A solid wedge line denotes a bond that sticks back behind the plane of the paper.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The compound with formula C4H9N has two degrees of unsaturation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Find the structure for 2,3,5,7-tetramethyloctane
A)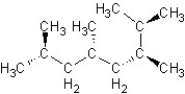
B)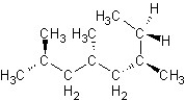
C)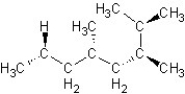
D)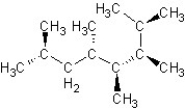
E)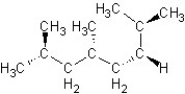
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Torsional or rotational energy is the difference in energy between the eclipsed and staggered conformations in alkane structure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
What is the correct IUPAC name for the following structure? 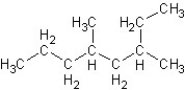
A)2-ethyl-3-methylheptane
B)3,5-dimethyloctane
C)4-methyl-6-ethylheptane
D)4,6-dimethyloctane
E)3,5-dimethyloctyl

A)2-ethyl-3-methylheptane
B)3,5-dimethyloctane
C)4-methyl-6-ethylheptane
D)4,6-dimethyloctane
E)3,5-dimethyloctyl

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following methods are used for the laboratory alkane synthesis?
I.catalytic addition of hydrogen to multiple carbon - carbon bonds
II.catalytic hydrogenation of aldehydes
III.from halogenated hydrocarbons and sodium
IV.from alkali metal salts of organic acids and alkali metal hydroxides
V.dehydrohalogenation of organic halogen compounds
A)I,II,and V
B)II,III,and V
C)I,III,and IV
D)II,III,and IV
E)III,IVand V
I.catalytic addition of hydrogen to multiple carbon - carbon bonds
II.catalytic hydrogenation of aldehydes
III.from halogenated hydrocarbons and sodium
IV.from alkali metal salts of organic acids and alkali metal hydroxides
V.dehydrohalogenation of organic halogen compounds
A)I,II,and V
B)II,III,and V
C)I,III,and IV
D)II,III,and IV
E)III,IVand V

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
What are skew conformations?
A)Skew conformations all possible conformations between the eclipsed and staggered extremes.
B)Skew conformations in which linear molecules get twisted.
C)Skew conformations are conformations that have the greatest energy.
D)Skew conformations are conformations that have the lowest energy.
E)Skew conformations are conformations that have the greatest energy barrier.
A)Skew conformations all possible conformations between the eclipsed and staggered extremes.
B)Skew conformations in which linear molecules get twisted.
C)Skew conformations are conformations that have the greatest energy.
D)Skew conformations are conformations that have the lowest energy.
E)Skew conformations are conformations that have the greatest energy barrier.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following structures represent the structure of trans-1-bromo-2-methylcyclohexane?
A)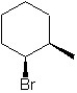
B)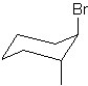
C)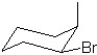
D)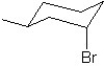
E)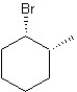
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Find the correct stereochemistries for the following four alkenes: 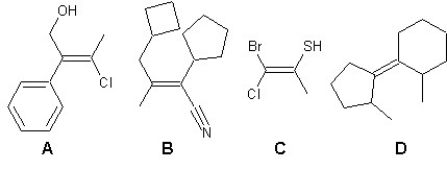
A)A is Z,B is Z,C is E,D is E
B)A is Z,B is E,C is Z,D is E
C)A is E,B is E,C is Z,D is E
D)A is E,B is E,C is Z,D is Z
E)A is Z,B is Z,C is E,D is Z

A)A is Z,B is Z,C is E,D is E
B)A is Z,B is E,C is Z,D is E
C)A is E,B is E,C is Z,D is E
D)A is E,B is E,C is Z,D is Z
E)A is Z,B is Z,C is E,D is Z

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Define "conformation."
A)Conformations are different arrangements of bonds in space.
B)Conformations are different structural representations of the same molecule.
C)Conformations are different spatial arrangements one structure can have.
D)Conformations are two enantiomers.
E)Conformations are all possible structural isomers for a given molecular formula.
A)Conformations are different arrangements of bonds in space.
B)Conformations are different structural representations of the same molecule.
C)Conformations are different spatial arrangements one structure can have.
D)Conformations are two enantiomers.
E)Conformations are all possible structural isomers for a given molecular formula.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Reforming is:
A)a process that joins low molecular mass hydrocarbons into higher molecular mass hydrocarbons
B)a process that converts straight chain hydrocarbons to branched hydrocarbons
C)a process that converts unsaturated organic compounds to saturated ones
D)a process that breaks down high molecular mass hydrocarbons into low molecular mass hydrocarbons
E)a process that converts branched hydrocarbons to straight chain hydrocarbons
A)a process that joins low molecular mass hydrocarbons into higher molecular mass hydrocarbons
B)a process that converts straight chain hydrocarbons to branched hydrocarbons
C)a process that converts unsaturated organic compounds to saturated ones
D)a process that breaks down high molecular mass hydrocarbons into low molecular mass hydrocarbons
E)a process that converts branched hydrocarbons to straight chain hydrocarbons

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following structures represents a structure of R isomer of 1-amino-2-propanol?
A)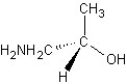
B)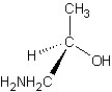
C)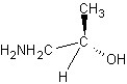
D)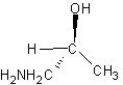
E)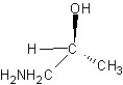
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
How many chiral carbon atoms can you find in the molecule below? 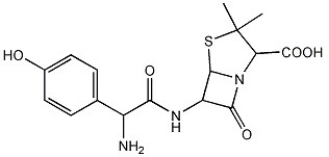
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
Which statements regarding chiral compounds are correct?
I."Chiral carbon" is synonymous with "asymmetric carbon."
II.The presence of one chiral carbon in a structure results in two possible enantiomers.
III.Two enantiomers can be interconverted without breaking any bonds.
IV.Two chiral molecules are mirror images of each other.
V.Two chiral molecules have significantly different chemical properties.
A)II,III,and V
B)I,II,and IV
C)I,III,and IV
D)II,IVand V
E)III,and V
I."Chiral carbon" is synonymous with "asymmetric carbon."
II.The presence of one chiral carbon in a structure results in two possible enantiomers.
III.Two enantiomers can be interconverted without breaking any bonds.
IV.Two chiral molecules are mirror images of each other.
V.Two chiral molecules have significantly different chemical properties.
A)II,III,and V
B)I,II,and IV
C)I,III,and IV
D)II,IVand V
E)III,and V

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following represent two different chair forms of methylcyclohexane?
A)
B)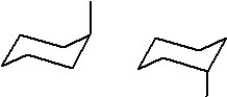
C)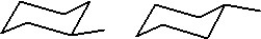
D)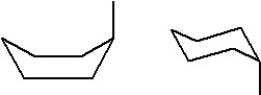
E)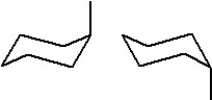
A)

B)

C)
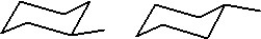
D)

E)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Give the name for CH3CH =CHCH3.
A)3-butene
B)2-butene
C)2-propene
D)2-pentene
E)2-butane
A)3-butene
B)2-butene
C)2-propene
D)2-pentene
E)2-butane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the INCORRECT statement.
A)In thermal cracking large hydrocarbon molecules are broken down into smaller molecules,ideally,molecules in the gasoline range.
B)Reforming converts straight-chain hydrocarbons into branched-chain hydrocarbons.
C)Branched-chain hydrocarbons have higher octane numbers.
D)Alkylation is the joining of small hydrocarbon fragments to larger molecules.
E)Engine knocking is caused by the smooth firing of gasoline.
A)In thermal cracking large hydrocarbon molecules are broken down into smaller molecules,ideally,molecules in the gasoline range.
B)Reforming converts straight-chain hydrocarbons into branched-chain hydrocarbons.
C)Branched-chain hydrocarbons have higher octane numbers.
D)Alkylation is the joining of small hydrocarbon fragments to larger molecules.
E)Engine knocking is caused by the smooth firing of gasoline.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
You found an organic compound containing chiral carbon atom with the following four substituents: HC≡C-,Cl-,O=C- and H2C=CH-.What would be the correct order of their priorities?
A)HC≡C- > O=C- > Cl- > H2C=CH-
B)Cl- > HC≡C- > O=C- > H2C=CH-
C)Cl- > O=C- > HC≡C- > H2C=CH-
D)Cl- > O=C-> H2C=CH- > HC≡C-
E)O=C- > Cl- > HC≡C- > H2C=CH-
A)HC≡C- > O=C- > Cl- > H2C=CH-
B)Cl- > HC≡C- > O=C- > H2C=CH-
C)Cl- > O=C- > HC≡C- > H2C=CH-
D)Cl- > O=C-> H2C=CH- > HC≡C-
E)O=C- > Cl- > HC≡C- > H2C=CH-

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
There are three rules for assigning priorities:
Rule 1: A substituent atom of higher atomic number takes precedence over one of lower atomic number.
Rule 2: If two substituent atoms attached to the stereocenter have the same priority,the atom on the right of a hydrogen atom bonded to chiral carbon is a substituent of higher priority.
Rule 3: Double and triple bonds are treated as if they were single,and the atoms in them are duplicated or triplicated at each end by the particular atoms at the other end of the multiple bond.
Which rule(s)is/are stated incorrectly?
A)rule 2
B)rules 1 and 3
C)rule 3
D)rules 2 and 3
E)rules 1 and 2
Rule 1: A substituent atom of higher atomic number takes precedence over one of lower atomic number.
Rule 2: If two substituent atoms attached to the stereocenter have the same priority,the atom on the right of a hydrogen atom bonded to chiral carbon is a substituent of higher priority.
Rule 3: Double and triple bonds are treated as if they were single,and the atoms in them are duplicated or triplicated at each end by the particular atoms at the other end of the multiple bond.
Which rule(s)is/are stated incorrectly?
A)rule 2
B)rules 1 and 3
C)rule 3
D)rules 2 and 3
E)rules 1 and 2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
Can 1,3,5-hexatriene be considered an aromatic compound? Why do you think so?
A)Yes,because it contains alternating double and single bonds.
B)No,because the molecule is not a flat ring.
C)Yes,because its double bonds contain 4n+2 electrons (n = 1).
D)No,because the structure is not based on benzene ring.
E)Yes,because the molecule has a delocalized π electron cloud.
A)Yes,because it contains alternating double and single bonds.
B)No,because the molecule is not a flat ring.
C)Yes,because its double bonds contain 4n+2 electrons (n = 1).
D)No,because the structure is not based on benzene ring.
E)Yes,because the molecule has a delocalized π electron cloud.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
Choose the INCORRECT statement.
A)Axial H atoms are directed above and below the cyclohexane ring.
B)A substitution reaction typically involves a functional group replacing a hydrogen atom on a chain or ring containing molecule.
C)A free radical is a compound with an unpaired electron.
D)LPG is linear polymerized gas.
E)Unsaturated usually means a double,triple or delocalized electron system is present in the molecule.
A)Axial H atoms are directed above and below the cyclohexane ring.
B)A substitution reaction typically involves a functional group replacing a hydrogen atom on a chain or ring containing molecule.
C)A free radical is a compound with an unpaired electron.
D)LPG is linear polymerized gas.
E)Unsaturated usually means a double,triple or delocalized electron system is present in the molecule.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Find the answer that has correct both the two structures of cis-1-isopropyl-4-methylcyclohexane and their relative stability (stability is indicated with ">" meaning "more stable than").
A)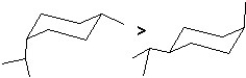
B)
C)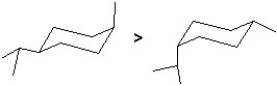
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
How many chiral carbon atoms can you find in the molecule below? 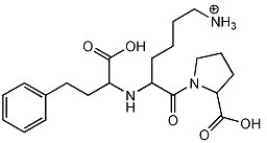
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Why does benzene have a much higher boiling point (80 °C)than hexane (69 °C),even though they have the same number of carbons?
A)Benzene has fewer hydrogens than hexane.
B)Benzene is much more polar than hexane which enhances the attractive forces between molecules and raises the boiling point.
C)Benzene is planar and has delocalized electron density which increases the attractive forces between molecules and raises the boiling point.
D)Hexane has more Kekulé structures than benzene.
E)Benzene can covalently bond to another benzene molecule which increases its boiling point.
A)Benzene has fewer hydrogens than hexane.
B)Benzene is much more polar than hexane which enhances the attractive forces between molecules and raises the boiling point.
C)Benzene is planar and has delocalized electron density which increases the attractive forces between molecules and raises the boiling point.
D)Hexane has more Kekulé structures than benzene.
E)Benzene can covalently bond to another benzene molecule which increases its boiling point.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Choose the best configuration assignments for the three double bonds,labeled A,B and C,in the following compound: 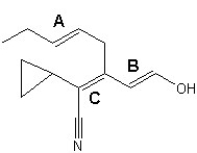
A)A is E,B is E,and C is E
B)A is E,B is E,and C is Z
C)A is Z,B is Z,and C is E
D)A is E,B is Z,and C is E
E)A is Z,B is E,and C is Z

A)A is E,B is E,and C is E
B)A is E,B is E,and C is Z
C)A is Z,B is Z,and C is E
D)A is E,B is Z,and C is E
E)A is Z,B is E,and C is Z

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
The diol,ethylene glycol,is used extensively as an antifreeze additive in automobile radiators.What properties of ethylene glycol make it a good antifreeze?
A)Ethylene glycol coats the radiator to prevent crystallization of ice at low temperatures.
B)Ethylene glycol has a higher boiling point than water and is soluble in water.
C)Ethylene glycol has a lower boiling point than water and is soluble in water.
D)Ethylene glycol forms a film on the surface of the water to prevent ice formation in the radiator.
E)Ethylene glycol will remain in the radiator after the water evaporates.This prevents freezing in cold climates.
A)Ethylene glycol coats the radiator to prevent crystallization of ice at low temperatures.
B)Ethylene glycol has a higher boiling point than water and is soluble in water.
C)Ethylene glycol has a lower boiling point than water and is soluble in water.
D)Ethylene glycol forms a film on the surface of the water to prevent ice formation in the radiator.
E)Ethylene glycol will remain in the radiator after the water evaporates.This prevents freezing in cold climates.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
What is the IUPAC name for the following molecule? 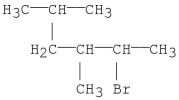
A)2-bromo-4-isopropyl-3-methylbutane
B)2-bromo-3,5-dimethylhexane
C)3,5-dimethyl-2-bromohexane
D)2-bromo-3-methyl-4-propylbutane
E)2-bromo-5,5,3-trimethylpentane

A)2-bromo-4-isopropyl-3-methylbutane
B)2-bromo-3,5-dimethylhexane
C)3,5-dimethyl-2-bromohexane
D)2-bromo-3-methyl-4-propylbutane
E)2-bromo-5,5,3-trimethylpentane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Compound A easily reacts with water to give compound B.Compound B can be oxidized to produce compound C.Both B and C can be further oxidized to produce D.To which classes of organic compounds A,B,C and D most likely belong to,respectively?
A)A is an alkane,B is an alcohol,C is an aldehyde and D is a carboxylic acid
B)A is an alkene,B is an ether,C is an aldehyde and D is a carboxylic acid
C)A is an alkene,B is an alcohol,C is a ketone and D is a carboxylic acid
D)A is an alkene,B is an alcohol,C is an aldehyde and D is an ester
E)A is an alkene,B is an alcohol,C is an aldehyde and D is a carboxylic acid
A)A is an alkane,B is an alcohol,C is an aldehyde and D is a carboxylic acid
B)A is an alkene,B is an ether,C is an aldehyde and D is a carboxylic acid
C)A is an alkene,B is an alcohol,C is a ketone and D is a carboxylic acid
D)A is an alkene,B is an alcohol,C is an aldehyde and D is an ester
E)A is an alkene,B is an alcohol,C is an aldehyde and D is a carboxylic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which class of compounds is the least reactive?
A)ethers
B)alcohols
C)ketones
D)alkyl halides
E)aldehydes
A)ethers
B)alcohols
C)ketones
D)alkyl halides
E)aldehydes

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
How many skeletal isomers for C6H14 can be drawn?
A)6
B)3
C)8
D)5
E)2
A)6
B)3
C)8
D)5
E)2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Name the functional groups in the molecule below: 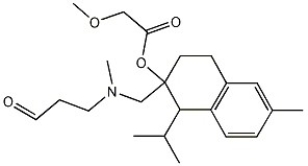
A)ester,ether,amine,keto
B)carboxyl,ether,amine,keto
C)ester,ether,amine,aldehyde
D)ester,ether,amide,aldehyde
E)ester,carboxyl,amine,keto

A)ester,ether,amine,keto
B)carboxyl,ether,amine,keto
C)ester,ether,amine,aldehyde
D)ester,ether,amide,aldehyde
E)ester,carboxyl,amine,keto

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
An alcohol of formula C3H8O in which there are only three different kinds of hydrogens is:
A)2-propanol
B)1-propanol
C)cyclopropanol
D)ethanol
E)None of the above
A)2-propanol
B)1-propanol
C)cyclopropanol
D)ethanol
E)None of the above

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
CH3(C=O)CH3 is a(n):
A)ether
B)ketone
C)ester
D)aldehyde
E)carboxylic acid
A)ether
B)ketone
C)ester
D)aldehyde
E)carboxylic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
Provide names for compounds A,B and C: 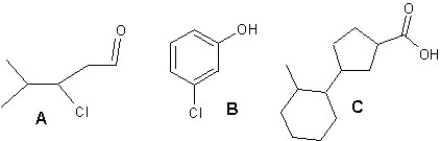
A)A is 3-chloro-4-methylpentanal,B is meta-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanecarboxylic acid
B)A is 3-chloro-4-methylpentanol,B is meta-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanecarboxylic acid
C)A is 3-chloro-4-methylpentanal,B is ortho-chlorophenol and C is 3-(3-methylcyclohexyl)cyclopentanecarboxylic acid
D)A is 3-chloro-4-methylpentanone,B is meta-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanal
E)A is 4-chloro-3-methylpentanal,B is para-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanecarboxylic acid

A)A is 3-chloro-4-methylpentanal,B is meta-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanecarboxylic acid
B)A is 3-chloro-4-methylpentanol,B is meta-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanecarboxylic acid
C)A is 3-chloro-4-methylpentanal,B is ortho-chlorophenol and C is 3-(3-methylcyclohexyl)cyclopentanecarboxylic acid
D)A is 3-chloro-4-methylpentanone,B is meta-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanal
E)A is 4-chloro-3-methylpentanal,B is para-chlorophenol and C is 3-(2-methylcyclohexyl)cyclopentanecarboxylic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
The terms ortho,meta and para are used to describe the substitution on benzene ring.What do they stand for?
A)Ortho stands for two adjacent substituents,meta for two substituents with one carbon between them and para for two substituents with two carbon atoms between them.
B)Ortho stands for two cis substituents,meta for two trans substituents and para for two opposite substituents.
C)Ortho stands for two adjacent substituents,meta for two different adjacent substituents and para for two substituents with one carbon atoms between them.
D)Ortho stands for two substituents with one carbon atom between them,meta for two substituents with two carbons between them and para for two substituents with three carbon atoms between them.
E)Ortho stands for two substituents with two carbon atoms between them,meta for two substituents with three carbons between them and para for two substituents with four carbon atoms between them.
A)Ortho stands for two adjacent substituents,meta for two substituents with one carbon between them and para for two substituents with two carbon atoms between them.
B)Ortho stands for two cis substituents,meta for two trans substituents and para for two opposite substituents.
C)Ortho stands for two adjacent substituents,meta for two different adjacent substituents and para for two substituents with one carbon atoms between them.
D)Ortho stands for two substituents with one carbon atom between them,meta for two substituents with two carbons between them and para for two substituents with three carbon atoms between them.
E)Ortho stands for two substituents with two carbon atoms between them,meta for two substituents with three carbons between them and para for two substituents with four carbon atoms between them.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the degree of unsaturation for the following molecules:
Molecule I: C3H4
Molecule II: C5H10
Molecule III: C4H6
A)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 1 and for molecule III the degree of unsaturation is 1.
B)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 0 and for molecule III the degree of unsaturation is 2.
C)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 1 and for molecule III the degree of unsaturation is 2.
D)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 2 and for molecule III the degree of unsaturation is 2.
E)For molecule I the degree of unsaturation is 1,for molecule II the degree of unsaturation is 2 and for molecule III the degree of unsaturation is 1.
Molecule I: C3H4
Molecule II: C5H10
Molecule III: C4H6
A)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 1 and for molecule III the degree of unsaturation is 1.
B)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 0 and for molecule III the degree of unsaturation is 2.
C)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 1 and for molecule III the degree of unsaturation is 2.
D)For molecule I the degree of unsaturation is 2,for molecule II the degree of unsaturation is 2 and for molecule III the degree of unsaturation is 2.
E)For molecule I the degree of unsaturation is 1,for molecule II the degree of unsaturation is 2 and for molecule III the degree of unsaturation is 1.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the INCORRECT statement.
A)CH3CH2CH2CH2CH2NH2 is an amine.
B)CH3CH2OCH2CH2CH3 is an ether.
C)CH3CH2CH2CH2CH2OH is an alcohol.
D)O CH3CH2CH2CH2CH is a ketone.
CH3CH2CH2CH2CH is a ketone.
E)CH3CH2CH2CH=CH2 is an alkene.
A)CH3CH2CH2CH2CH2NH2 is an amine.
B)CH3CH2OCH2CH2CH3 is an ether.
C)CH3CH2CH2CH2CH2OH is an alcohol.
D)O
 CH3CH2CH2CH2CH is a ketone.
CH3CH2CH2CH2CH is a ketone.E)CH3CH2CH2CH=CH2 is an alkene.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
What are heterocyclic compounds?
A)They are cyclic compounds in which carbon has one or more substituents that are neither hydrogen or another carbon atom.
B)They are cyclic compounds in which two or more carbon rings are connected by C-C bonds (for example biphenyl).
C)They are cyclic compounds in which carbon ring is connected to another ring not containing carbon atoms.
D)They are cyclic compounds in which one or more atoms are not carbon atoms.
E)They are cyclic compounds that can have more than one stable conformation.
A)They are cyclic compounds in which carbon has one or more substituents that are neither hydrogen or another carbon atom.
B)They are cyclic compounds in which two or more carbon rings are connected by C-C bonds (for example biphenyl).
C)They are cyclic compounds in which carbon ring is connected to another ring not containing carbon atoms.
D)They are cyclic compounds in which one or more atoms are not carbon atoms.
E)They are cyclic compounds that can have more than one stable conformation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following synthetic procedures are plausible?
I.synthesis of esters from alcohols and acids
II.synthesis of ethers from acids and alcohols
III.synthesis of amides from acids and amines
IV.synthesis of alcohols from alkanes and water
V.synthesis of aldehydes from alcohols and oxidizing agents
A)I,III,and IV
B)I,III,and V
C)II,III,and V
D)II,III,and IV
E)III,IV,and V
I.synthesis of esters from alcohols and acids
II.synthesis of ethers from acids and alcohols
III.synthesis of amides from acids and amines
IV.synthesis of alcohols from alkanes and water
V.synthesis of aldehydes from alcohols and oxidizing agents
A)I,III,and IV
B)I,III,and V
C)II,III,and V
D)II,III,and IV
E)III,IV,and V

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
C3H8O has how many skeletal isomers?
A)2
B)4
C)5
D)6
E)3
A)2
B)4
C)5
D)6
E)3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Which two simple functional groups make carboxyl group?
A)aldehyde and keto groups
B)aldehyde and hydroxyl groups
C)keto and hydroxyl groups
D)ester and hydroxyl groups
E)ester and ether groups
A)aldehyde and keto groups
B)aldehyde and hydroxyl groups
C)keto and hydroxyl groups
D)ester and hydroxyl groups
E)ester and ether groups

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
To produce an ether from methanol,one can use:
A)reacting alkene with water in a presence of a strong mineral acid
B)alcohol and concentrated H2SO4
C)KOH in alcohol
D)Cu,heat
E)reacting alcohol with sodium
A)reacting alkene with water in a presence of a strong mineral acid
B)alcohol and concentrated H2SO4
C)KOH in alcohol
D)Cu,heat
E)reacting alcohol with sodium

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
What is the degree of unsaturation for the following compounds? 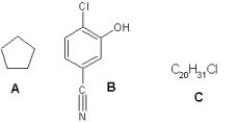
A)For A the degree of unsaturation is 1,for B is 6 and for C is 5.
B)For A the degree of unsaturation is 1,for B is 5 and for C is 6.
C)For A the degree of unsaturation is 0,for B is 6 and for C is 5.
D)For A the degree of unsaturation is 1,for B is 6 and for C is 4.
E)For A the degree of unsaturation is 1,for B is 4 and for C is 5.

A)For A the degree of unsaturation is 1,for B is 6 and for C is 5.
B)For A the degree of unsaturation is 1,for B is 5 and for C is 6.
C)For A the degree of unsaturation is 0,for B is 6 and for C is 5.
D)For A the degree of unsaturation is 1,for B is 6 and for C is 4.
E)For A the degree of unsaturation is 1,for B is 4 and for C is 5.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which class of compounds is most oxidized?
A)alkanes
B)alcohols
C)carboxylic acids
D)aldehydes
E)ketones
A)alkanes
B)alcohols
C)carboxylic acids
D)aldehydes
E)ketones

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which methods can be used to prepare alcohols?
I.addition of H2O to alkenes in the presence of H2SO4
II.reacting alkanes with a concentrated solution of CH3OH
III.hydrolysis of alkyl halides
IV.methanol can be prepared from CO(g)and H2(g)in the presence of a heterogeneous catalyst
V.removing one oxygen atom from a carboxylic acid
A)I,IIand IV
B)I,III,and IV
C)II,III,and V
D)I,II,and V
E)II,IVand V
I.addition of H2O to alkenes in the presence of H2SO4
II.reacting alkanes with a concentrated solution of CH3OH
III.hydrolysis of alkyl halides
IV.methanol can be prepared from CO(g)and H2(g)in the presence of a heterogeneous catalyst
V.removing one oxygen atom from a carboxylic acid
A)I,IIand IV
B)I,III,and IV
C)II,III,and V
D)I,II,and V
E)II,IVand V

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Give the condensed structural formula for 3-bromopentane.
A)CH3CH2CHBrCH2CH2CH3
B)CH3CHBrCH2CH2CH3
C)(CH3)3CHBrCH2CH3
D)CH3CH2CHBrCH2CH3
E)CH3CH2CBr3CH2CH3
A)CH3CH2CHBrCH2CH2CH3
B)CH3CHBrCH2CH2CH3
C)(CH3)3CHBrCH2CH3
D)CH3CH2CHBrCH2CH3
E)CH3CH2CBr3CH2CH3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
The burning of alkanes to produce carbon dioxide and water is known as what type of reaction?
A)thermal cracking
B)reforming
C)radical substitution
D)oxidation
E)alkylation
A)thermal cracking
B)reforming
C)radical substitution
D)oxidation
E)alkylation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
The two benzene substituents of the common analgesic shown below are considered in what relation to one another? 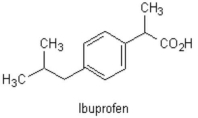
A)ortho
B)para
C)meta
D)trans
E)1,3

A)ortho
B)para
C)meta
D)trans
E)1,3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
What is the condensed structural formula for 2-pentyne?
A)CH2 CHCH2CH2CH3
CHCH2CH2CH3
B)CH3C CCH2CH3
CCH2CH3
C)CH3CH CHCH2CH3
CHCH2CH3
D)CH3C CCH3
CCH3
E)CH3CH CCH2CH2CH3
CCH2CH2CH3
A)CH2
 CHCH2CH2CH3
CHCH2CH2CH3 B)CH3C
 CCH2CH3
CCH2CH3 C)CH3CH
 CHCH2CH3
CHCH2CH3 D)CH3C
 CCH3
CCH3 E)CH3CH
 CCH2CH2CH3
CCH2CH2CH3 
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following has the lowest melting point: cyclohexane,hexane,benzene?
A)cyclohexane
B)hexane
C)benzene
D)They have approximately the same melting point.
E)There is not enough information to determine.
A)cyclohexane
B)hexane
C)benzene
D)They have approximately the same melting point.
E)There is not enough information to determine.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
What are the possible positions for hydrogen on the chair conformation of cyclohexane?
A)staggered and eclipsed
B)axial and equatorial
C)equatorial and apical
D)longitudinal and latitudinal
E)chair and boat
A)staggered and eclipsed
B)axial and equatorial
C)equatorial and apical
D)longitudinal and latitudinal
E)chair and boat

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following chiral compounds is(are)R enantiomer(s)? 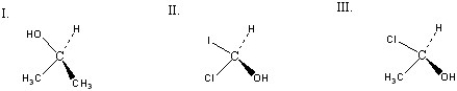
A)I
B)II
C)III
D)II and III
E)I and II

A)I
B)II
C)III
D)II and III
E)I and II

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following compounds is(are)chiral? 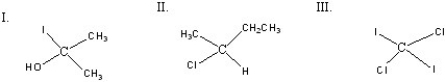
A)I
B)II
C)III
D)I and II
E)II and III

A)I
B)II
C)III
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds is(are)chiral? 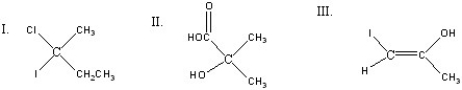
A)I
B)II
C)III
D)I and II
E)II and III

A)I
B)II
C)III
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Combustion of 493 mg of a hydrocarbon gave 1549 mg CO2 and 633.9 mg H2O.What is the molecular formula if the molecular weight is subsequently found to be 42?
A)CO2
B)CH2
C)C6H12
D)C2H2O
E)C3H6
A)CO2
B)CH2
C)C6H12
D)C2H2O
E)C3H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
If an ethyl group is substituted for one hydrogen of cyclohexane,what would be the preferred position of that ethyl substituent on the ring?
A)axial position
B)equatorial position
C)chair position
D)boat position
E)twist boat conformation
A)axial position
B)equatorial position
C)chair position
D)boat position
E)twist boat conformation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Give the condensed structural formula for 3-methyl-3-hexene.
A)CH3CH2CH(CH3)CH=CHCH3
B)CH3CH2C(CH3)2CH=CHCH3
C)(CH3)3CCH=CHCH3
D)CH3CH2C(CH3)=CHCH2CH3
E)(CH3)6CCH2CH=CH2
A)CH3CH2CH(CH3)CH=CHCH3
B)CH3CH2C(CH3)2CH=CHCH3
C)(CH3)3CCH=CHCH3
D)CH3CH2C(CH3)=CHCH2CH3
E)(CH3)6CCH2CH=CH2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following chiral compounds is(are)S enantiomer(s)? 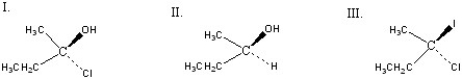
A)I
B)II
C)III
D)I and II
E)I and III

A)I
B)II
C)III
D)I and II
E)I and III

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
C4H10O has how many skeletal isomers?
A)11
B)12
C)8
D)7
E)6
A)11
B)12
C)8
D)7
E)6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following compounds is(are)E configuration(s)? 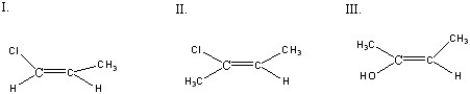
A)I
B)II
C)III
D)I + II
E)II + III

A)I
B)II
C)III
D)I + II
E)II + III

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
Give the name for CH3CH2CHBrCH3.
A)2-bromobutane
B)3-bromobutane
C)2-bromopropane
D)3-bromopropane
E)3-bromylpentane
A)2-bromobutane
B)3-bromobutane
C)2-bromopropane
D)3-bromopropane
E)3-bromylpentane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
How many different structural isomers are there of the compound dichlorobutane?
A)6
B)8
C)9
D)5
E)7
A)6
B)8
C)9
D)5
E)7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following compounds is(are)Z configuration(s)? 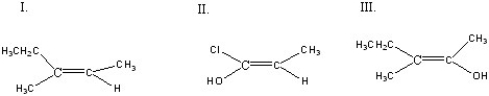
A)I
B)II
C)III
D)I + II
E)I + II + III

A)I
B)II
C)III
D)I + II
E)I + II + III

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
What is the complete systematic name for the following molecule? 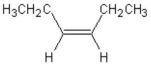
A)3-pentene
B)3-hexene
C)cis-3-hexene
D)trans-3-hexene
E)cis-3-hexyne

A)3-pentene
B)3-hexene
C)cis-3-hexene
D)trans-3-hexene
E)cis-3-hexyne

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Provide IUPAC names for the following compounds: 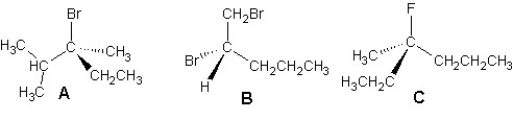
A)A is (3S)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3R)-3-fluoro-3-methylhexane
B)A is (3R)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3R)-3-fluoro-3-methylhexane
C)A is (3S)-3-bromo-2,3-dimethylpentane,B is (2R)-1,2-dibromopentane and C is (3R)-3-fluoro-3-methylhexane
D)A is (3S)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3S)-3-fluoro-3-methylhexane
E)A is (3R)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3S)-3-fluoro-3-methylhexane

A)A is (3S)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3R)-3-fluoro-3-methylhexane
B)A is (3R)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3R)-3-fluoro-3-methylhexane
C)A is (3S)-3-bromo-2,3-dimethylpentane,B is (2R)-1,2-dibromopentane and C is (3R)-3-fluoro-3-methylhexane
D)A is (3S)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3S)-3-fluoro-3-methylhexane
E)A is (3R)-3-bromo-2,3-dimethylpentane,B is (2S)-1,2-dibromopentane and C is (3S)-3-fluoro-3-methylhexane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


