Deck 2: Molecular Representations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/168
Play
Full screen (f)
Deck 2: Molecular Representations
1
Which of the following bond-line structures are of the same compound? 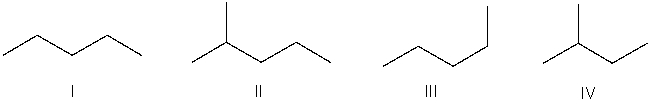
A) I and III
B) II and III
C) III and IV
D) II and IV
E) None of these

A) I and III
B) II and III
C) III and IV
D) II and IV
E) None of these
I and III
2
Which of the following is the correct Lewis structure for CH3(CH2)2NH2? 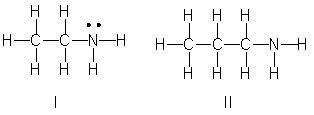
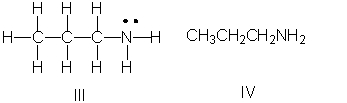
A) I
B) II
C) III
D) IV
E) Both II and III

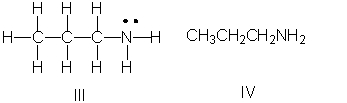
A) I
B) II
C) III
D) IV
E) Both II and III
III
3
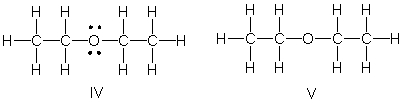
Identify the partially condensed structure for CH3CH2OCH2CH3. 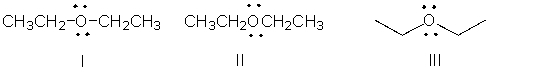
A) I
B) II
C) III
D) IV
E) V
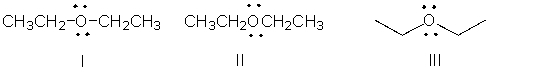

A) I
B) II
C) III
D) IV
E) V
I
4
Which of the following is the correct Lewis structure for CH3(CH2)2OH? 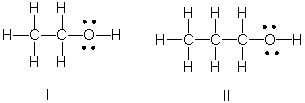
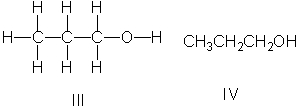
A) I
B) II
C) III
D) IV
E) Both II and III


A) I
B) II
C) III
D) IV
E) Both II and III

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the correct condensed structure for the following compound. 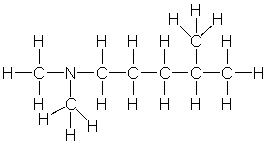


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the correct condensed structure for the following compound? 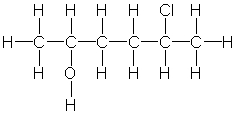
A) CH3CHOHCH2CHClCH3
B) CH3CHOH(CH2)2CHClCH3
C) (CH3)2CHOHCH2CH2Cl
D) HOCH3CHCH2CH2CH3CHCl
E) CH3C2H4CH3OHCl

A) CH3CHOHCH2CHClCH3
B) CH3CHOH(CH2)2CHClCH3
C) (CH3)2CHOHCH2CH2Cl
D) HOCH3CHCH2CH2CH3CHCl
E) CH3C2H4CH3OHCl

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the correct molecular formula for (CH3CH2)4C?
A) C8H20
B) C5H20
C) C9H20
D) C6H5
E) C3H20
A) C8H20
B) C5H20
C) C9H20
D) C6H5
E) C3H20

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
8
Provide the correct condensed structure for the following compound. 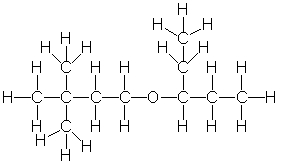


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the correct Lewis structure for (CH3)2CHCH2OH? 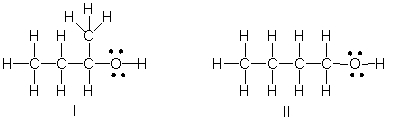
A) I
B) II
C) III
D) IV
E) Both III and IV


A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the correct condensed structure for the following compound? 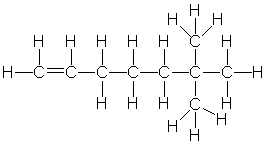
A) CH2=CH(CH2)3C(CH3)3
B) CH(CH2)4C(CH3)3
C) (CH3)2CH(CH2) 4CH3
D) CH2CH(CH2)3C(CH3)3
E) (CH)3(CH2)3C(CH3)3

A) CH2=CH(CH2)3C(CH3)3
B) CH(CH2)4C(CH3)3
C) (CH3)2CH(CH2) 4CH3
D) CH2CH(CH2)3C(CH3)3
E) (CH)3(CH2)3C(CH3)3

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the partially condensed structure for CH3CH2CH2NH2.
A)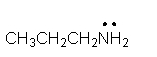
B)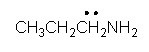
C)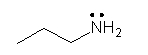
D)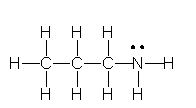
E)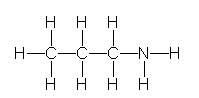
A)
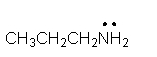
B)
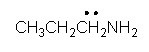
C)
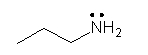
D)

E)


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the correct Lewis structure for (CH3)3C(CH2)2NHCH3? 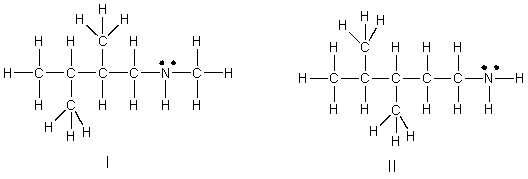
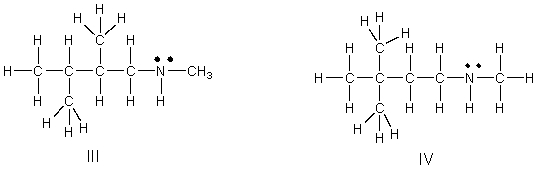
A) I
B) II
C) III
D) IV
E) V



A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
13
What is the molecular formula for the following compound? 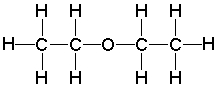
A) C2H6O
B) C4H6O
C) C4H10O
D) C2H4O
E) None of these

A) C2H6O
B) C4H6O
C) C4H10O
D) C2H4O
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds have a molecular formula of C2H6O? 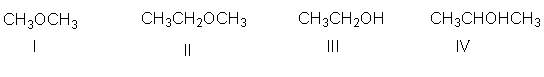
A) I
B) II
C) III
D) IV
E) Both I and III
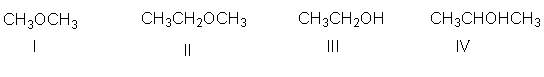
A) I
B) II
C) III
D) IV
E) Both I and III

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the correct condensed structure for the following compound? 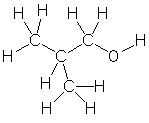
A) CH3CHCH3CH2OH
B) CH3CH2CH2OH
C) (CH3)2CHCH2OH
D) CH3CH2CH2OCH3
E) CH3CH3CHCH2OH

A) CH3CHCH3CH2OH
B) CH3CH2CH2OH
C) (CH3)2CHCH2OH
D) CH3CH2CH2OCH3
E) CH3CH3CHCH2OH

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
16
Draw the Lewis structure for (CH3)3C(CH2)2OCH(CH2CH3)2.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the correct condensed structure for the following compound? 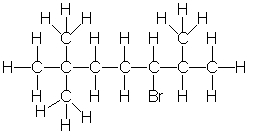
A) CH3C(CH3)2(CH2)2(CH)BrC(CH3)2
B) CH3CH3CH3C(CH2)2C(CH3)2CHBr
C) (CH3)3C(CH2)3BrCHCH3CH3
D) CH3CH3CH3C(CH2)2CHBrCHCH3CH3
E) (CH3)3C(CH2)2CHBrCH(CH3)2

A) CH3C(CH3)2(CH2)2(CH)BrC(CH3)2
B) CH3CH3CH3C(CH2)2C(CH3)2CHBr
C) (CH3)3C(CH2)3BrCHCH3CH3
D) CH3CH3CH3C(CH2)2CHBrCHCH3CH3
E) (CH3)3C(CH2)2CHBrCH(CH3)2

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
18
Draw the Lewis structure for CH3C≡C(CH2)3C(CH3)3.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the correct condensed structure for the following compound? 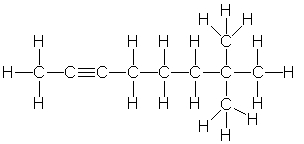
A) CH3C2(CH2)3C(CH3)3
B) CH3CC(CH2)3C(CH3)2CH3
C) (CH3)3C2(CH2)3CH3
D) CH3C≡C(CH2)3C(CH3)3
E) CH3CC(CH2)3C(CH3)3

A) CH3C2(CH2)3C(CH3)3
B) CH3CC(CH2)3C(CH3)2CH3
C) (CH3)3C2(CH2)3CH3
D) CH3C≡C(CH2)3C(CH3)3
E) CH3CC(CH2)3C(CH3)3

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following bond-line structures are of the same compound? 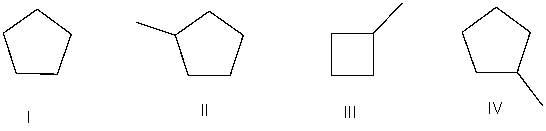
A) I and II
B) II and III
C) III and IV
D) II and IV
E) None of these

A) I and II
B) II and III
C) III and IV
D) II and IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
21
How many hydrogen atoms are connected to the indicated carbon atom? 
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the correct bond-line structure for CH3C≡C(CH2)2CH(CH3)2? 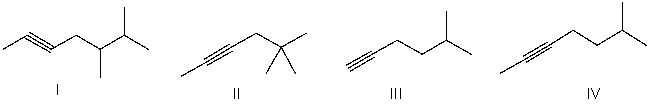
A) I
B) II
C) III
D) IV
E) None of these
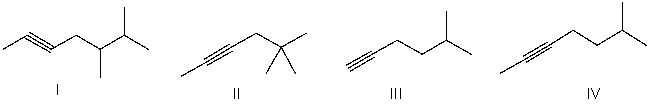
A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
23
For the following equation, how many hydrogen atoms are added or lost? 
A) added one
B) added two
C) lost one
D) lost two
E) no change

A) added one
B) added two
C) lost one
D) lost two
E) no change

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
24
How many hydrogen atoms are connected to the indicated carbon atom? 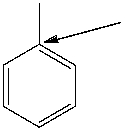
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
25
For the following equation, how many hydrogen atoms are added or lost? 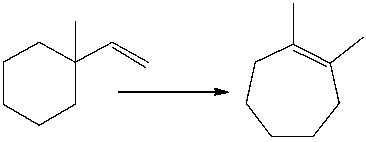
A) added one
B) added two
C) lost one
D) lost two
E) no change

A) added one
B) added two
C) lost one
D) lost two
E) no change

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
26
For the following equation, how many hydrogen atoms are added or lost? 
A) added one
B) added two
C) lost one
D) lost two
E) no change

A) added one
B) added two
C) lost one
D) lost two
E) no change

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
27
For the following equation, how many hydrogen atoms are added or lost? 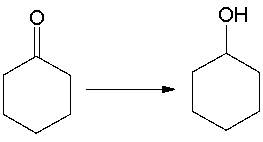
A) added one
B) added two
C) lost one
D) lost two
E) no change

A) added one
B) added two
C) lost one
D) lost two
E) no change

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
28
How many hydrogen atoms are connected to the indicated carbon atom? 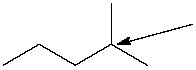
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the correct Lewis structure for the following compound? 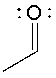
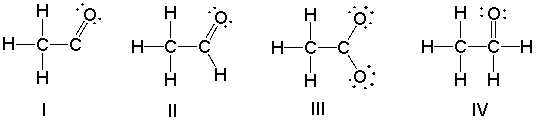
A) I
B) II
C) III
D) IV
E) none of these
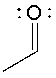

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the correct bond-line structure for (CH3)2CHCH2C(CH3)3? 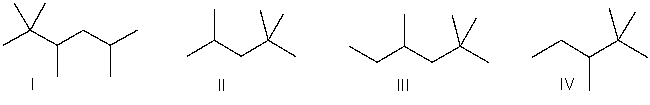
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
31
Draw a bond-line structure for CH3CH2O(CH2)2CH(CH3)2.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
32
How many hydrogen atoms are connected to the indicated carbon atom? 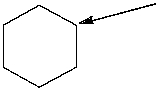
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the correct bond-line structure for CH3CHOH(CH2)2CH(CH2CH3) 2? 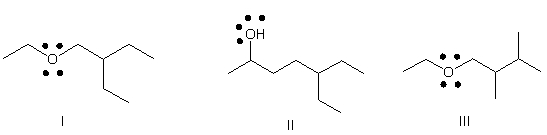
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
34
Draw a bond-line structure for (CH3)2N(CH2)3CH(CH3)2.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
35
For the following equation, how many hydrogen atoms are added or lost? 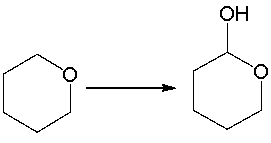
A) added one
B) added two
C) lost one
D) lost two
E) no change

A) added one
B) added two
C) lost one
D) lost two
E) no change

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
36
Draw a bond-line structure for CH3C≡C(CH2)3C(CH3)2CH2OCH3.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the correct bond-line structure for (CH3)4C? 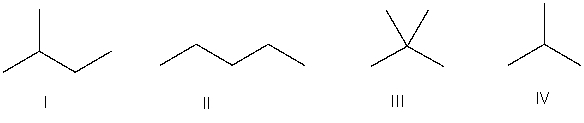
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
38
How many hydrogen atoms are connected to the indicated carbon atom? 
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the correct bond-line structure for (CH3)2CHCH2CH3? 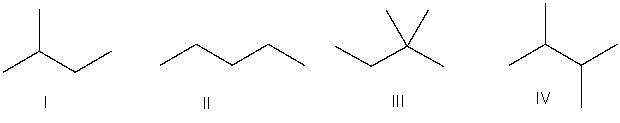
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
40
For the following equation, how many hydrogen atoms are added or lost? 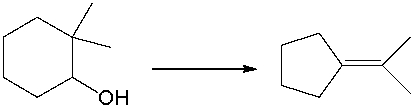
A) added one
B) added two
C) lost one
D) lost two
E) no change

A) added one
B) added two
C) lost one
D) lost two
E) no change

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds contain a ketone functional group? 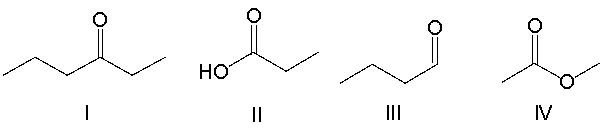
A) I
B) II
C) III
D) IV
E) All of these

A) I
B) II
C) III
D) IV
E) All of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
42
Draw a bond-line structure for each constitutional isomer with a molecular formula of C3H8O.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following compounds contain an alkyne functional group? 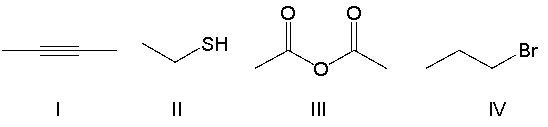
A) I
B) II
C) III
D) IV
E) none of the above

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following compounds contain an anhydride functional group? 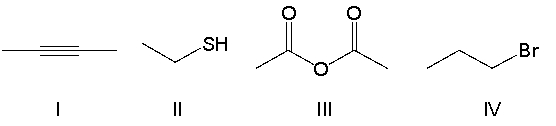
A) I
B) II
C) III
D) IV
E) none of the above

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
45
Naproxen, sold under the trade name Aleve, has the following structure. What is the molecular formula for naproxen? 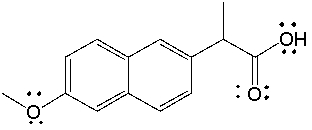


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
46
Draw a bond-line structure for each constitutional isomer with molecular formula C4H11N.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
47
Draw a bond-line structure for each constitutional isomer with a molecular formula of C2H4O.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following compounds contain an alcohol functional group? 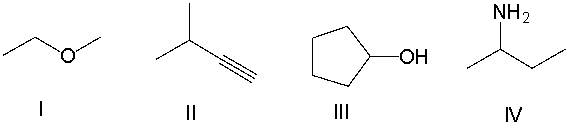
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
49
AZT, used in the treatment of AIDS, has the following structure. What is the molecular formula for AZT? 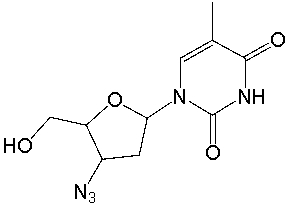


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
50
Provide a condensed structure for the following compound. 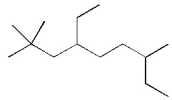


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
51
Provide a condensed structure for the following compound. 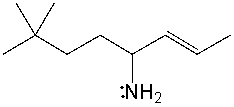


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following compounds contain an aromatic ring? 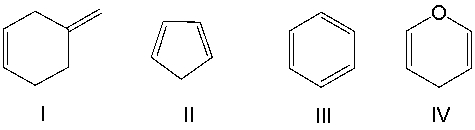
A) I
B) II
C) III
D) IV
E) Both III and IV

A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds contain an ester functional group? 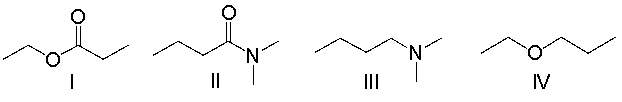
A) I
B) II
C) III
D) IV
E) Both I and IV

A) I
B) II
C) III
D) IV
E) Both I and IV

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds contain an alkyl halide functional group? 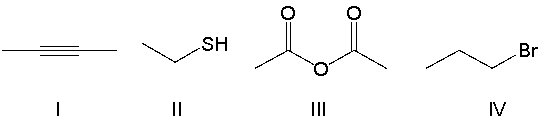
A) I
B) II
C) III
D) IV
E) none of the above

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following compounds contain an alkene functional group? 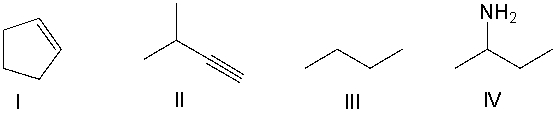
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds contain an amine functional group? 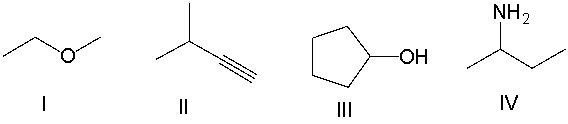
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds contain a thiol functional group? 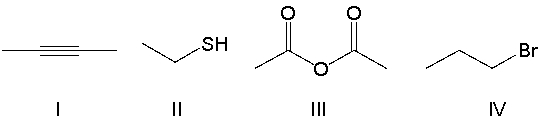
A) I
B) II
C) III
D) IV
E) none of the above

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
58
Draw a bond-line structure for each constitutional isomer with molecular formula C4H10O.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
59
Capsaicin, found in peppers, has the following structure. What is the molecular formula for capsaicin? 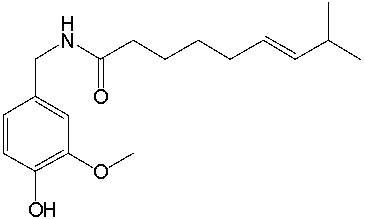


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following compounds contain an amide functional group? 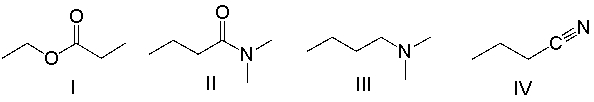
A) I
B) II
C) III
D) IV
E) Both II and III

A) I
B) II
C) III
D) IV
E) Both II and III

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
61
How many hydrogen atoms are connected to the indicated carbon atom? 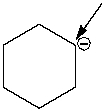
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
62
What is the formal charge on the indicated carbon atom? 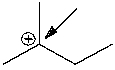
A) -2
B) -1
C) 0
D) +1
E) +2

A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
63
Amoxicillin, an antibiotic, has the following structure. Identify the functional groups in amoxicillin. 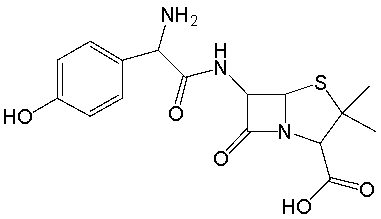


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
64
Tamiflu, the most effective antiviral drug used to treat avian influenza, has the following structure. Identify the functional groups in Tamiflu. 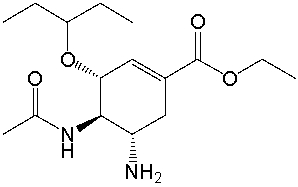


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
65
What functional group(s) is (are) present in the following compound? 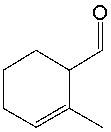
A) ketone and alkene
B) ketone and alkyne
C) aldehyde and alkene
D) aldehyde and alkyne
E) ester and alkene

A) ketone and alkene
B) ketone and alkyne
C) aldehyde and alkene
D) aldehyde and alkyne
E) ester and alkene

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
66
Draw all the constitutional isomers with a molecular formula of C3H6O and label the functional groups in each isomer.

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
67
How many hydrogen atoms are connected to the indicated carbon atom? 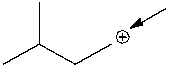
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
68
Viracept, used in the treatment of HIV, has the following structure. Identify the functional groups in Viracept. 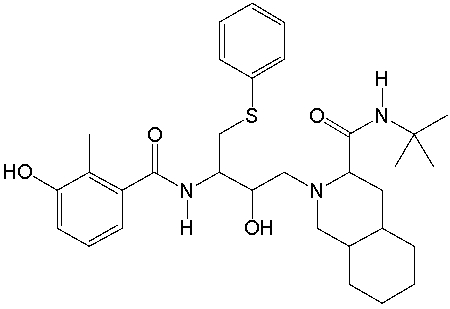


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
69
What is the formal charge on the indicated carbon atom? 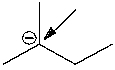
A) -2
B) -1
C) 0
D) +1
E) +2

A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
70
What is the formal charge on the indicated carbon atom? 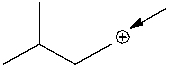
A) -2
B) -1
C) 0
D) +1
E) +2

A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
71
How many hydrogen atoms are connected to the indicated carbon atom? 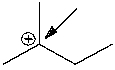
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
72
Identify the functional groups in the following compound. 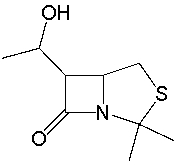


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
73
What is the formal charge on the indicated carbon atom? 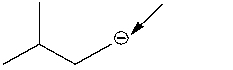
A) -2
B) -1
C) 0
D) +1
E) +2

A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
74
How many hydrogen atoms are connected to the indicated carbon atom? 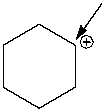
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
75
Aspartame, an artificial sweetener used in Equal and diet beverages, has the following structure. Identify the functional groups in Aspartame. 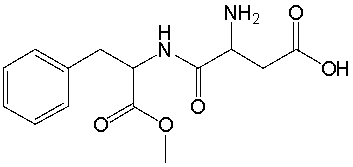


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
76
How many hydrogen atoms are connected to the indicated carbon atom? 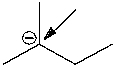
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds have both a ketone and an ester functional group? 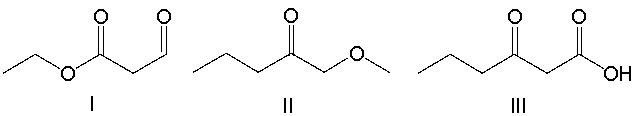
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
78
What is the formal charge on the indicated carbon atom? 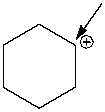
A) -2
B) -1
C) 0
D) +1
E) +2

A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
79
How many hydrogen atoms are connected to the indicated carbon atom? 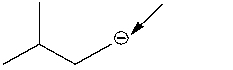
A) one
B) two
C) three
D) four
E) none

A) one
B) two
C) three
D) four
E) none

Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck
80
Norethynodrel, a component of the first combined oral contraceptive, has the following structure. Identify the functional groups in Norethynodrel. 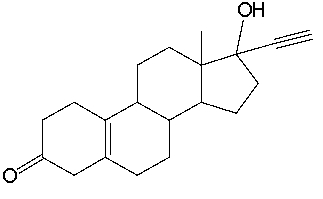


Unlock Deck
Unlock for access to all 168 flashcards in this deck.
Unlock Deck
k this deck


