Deck 7: Alkyl Halides: Nucleophilic Substitution and Elimination Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/224
Play
Full screen (f)
Deck 7: Alkyl Halides: Nucleophilic Substitution and Elimination Reactions
1
Which of the following is a primary alkyl halide?
A) (CH3)2CHCH2Cl
B) (CH3)2CClCH2CH3
C) (CH3)2CHCHClCH3
D) (CH3)2CHCH2CCl(CH3)2
A) (CH3)2CHCH2Cl
B) (CH3)2CClCH2CH3
C) (CH3)2CHCHClCH3
D) (CH3)2CHCH2CCl(CH3)2
(CH3)2CHCH2Cl
2
What is the IUPAC name for the following compound? 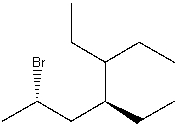
A) 2-Bromo-4-pentylhexane
B) (2S,4S)-2-Bromo-4,5-diethylheptane
C) 3,4-Diethyl-6-bromoheptane
D) 2-Bromo-4-methylhexane
E) (2R,4R)-2-Bromo-4,5-diethylheptane

A) 2-Bromo-4-pentylhexane
B) (2S,4S)-2-Bromo-4,5-diethylheptane
C) 3,4-Diethyl-6-bromoheptane
D) 2-Bromo-4-methylhexane
E) (2R,4R)-2-Bromo-4,5-diethylheptane
(2S,4S)-2-Bromo-4,5-diethylheptane
3
For the following reaction, label the nucleophile, electrophile, and leaving group. 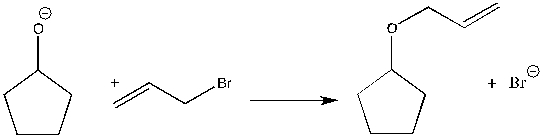

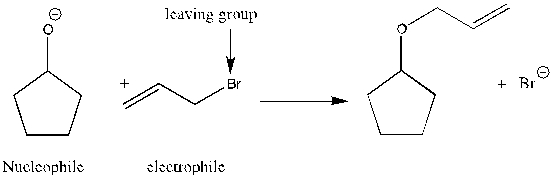
4
What is the nucleophile in the following reaction? 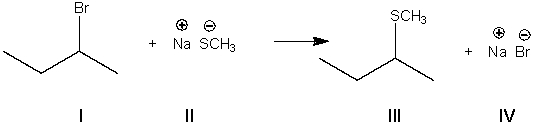
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
5
What is the nucleophile in the following reaction? 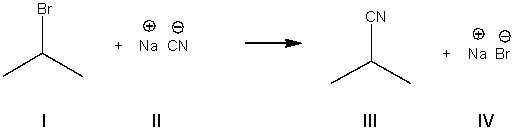
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a substitution reaction? 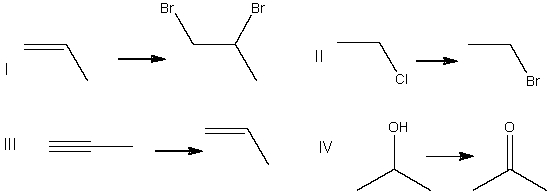
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is an elimination reaction? 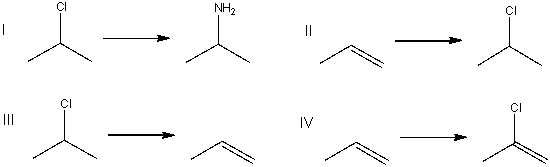
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is an elimination reaction? 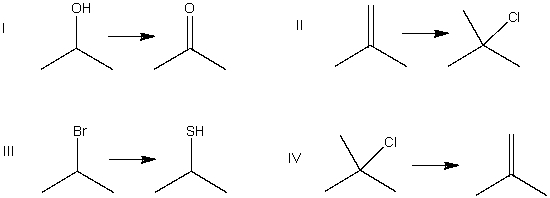
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a tertiary alkyl halide?
A) 1-Bromo-2-methylpropane
B) 2-Bromopropane
C) 1-Bromobutane
D) 2-Bromo-2-methylpropane
A) 1-Bromo-2-methylpropane
B) 2-Bromopropane
C) 1-Bromobutane
D) 2-Bromo-2-methylpropane

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a secondary alkyl halide?
A) 1-Bromo-2-methylpropane
B) 2-Bromopropane
C) 1-Bromobutane
D) 2-Bromo-2-methylpropane
A) 1-Bromo-2-methylpropane
B) 2-Bromopropane
C) 1-Bromobutane
D) 2-Bromo-2-methylpropane

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
11
What is the electrophile in the following reaction? 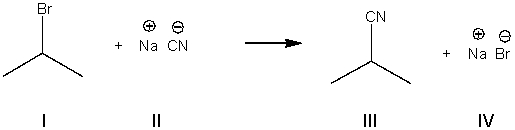
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
12
What is the electrophile in the following reaction? 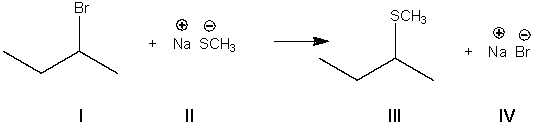
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a tertiary alkyl halide?
A) (CH3)2CHCH2Cl
B) (CH3)2CClCH2CH3
C) (CH3)2CHCHClCH3
D) (CH3)2CHCH2CHClCH2CH3
A) (CH3)2CHCH2Cl
B) (CH3)2CClCH2CH3
C) (CH3)2CHCHClCH3
D) (CH3)2CHCH2CHClCH2CH3

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name for the following compound? 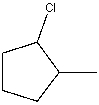
A) Chlorocyclopentane
B) 2-Chloro-1-methylcyclopentane
C) 1-Methyl-2-chlorocyclopentane
D) 1-Chloro-2-methylcyclopentane

A) Chlorocyclopentane
B) 2-Chloro-1-methylcyclopentane
C) 1-Methyl-2-chlorocyclopentane
D) 1-Chloro-2-methylcyclopentane

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a substitution reaction? 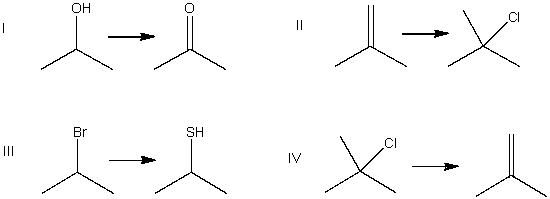
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name for the following compound? 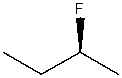
A) 3-Fluorobutane
B) 2-Fluorobutane
C) (S)-2-Fluorobutane
D) (R)-2-Fluorobutane

A) 3-Fluorobutane
B) 2-Fluorobutane
C) (S)-2-Fluorobutane
D) (R)-2-Fluorobutane

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
17
For the following reaction, label the nucleophile, electrophile, and leaving group. 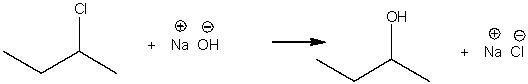
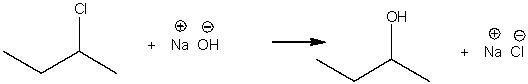

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a substitution reaction? 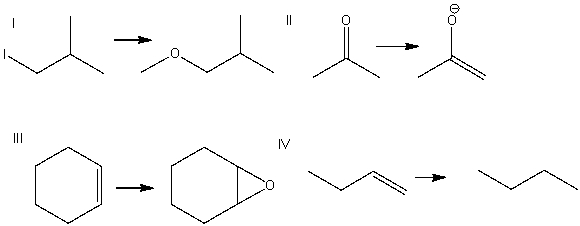
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a secondary alkyl halide?
A) (CH3)2CHCH2Cl
B) (CH3)2CClCH2CH3
C) (CH3)2CHCHClCH3
D) (CH3)2CHCH2CCl(CH3)2
A) (CH3)2CHCH2Cl
B) (CH3)2CClCH2CH3
C) (CH3)2CHCHClCH3
D) (CH3)2CHCH2CCl(CH3)2

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
20
What is the classification for the following halide? 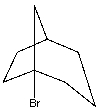
A) primary halide
B) secondary halide
C) tertiary halide
D) quaternary halide

A) primary halide
B) secondary halide
C) tertiary halide
D) quaternary halide

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the structure for (4R,8S)-4-iodo-2,2,8-trimethyldecane.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
22
When drawing a curved arrow mechanism, the tail of the arrow starts at______.
A) the bond that is being formed
B) the atom with the positive charge
C) the source of electrons that is being moved
D) the location to which the electrons are being moved
A) the bond that is being formed
B) the atom with the positive charge
C) the source of electrons that is being moved
D) the location to which the electrons are being moved

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name for the following compound? 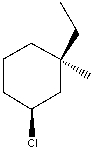


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the following SN2 reaction, 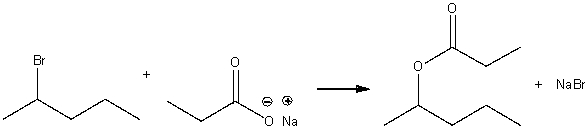 Assuming no other changes, what is the effect on the rate, if the concentration of 2-bromopentane is doubled and the concentration of CH3CH2COONa is halved?
Assuming no other changes, what is the effect on the rate, if the concentration of 2-bromopentane is doubled and the concentration of CH3CH2COONa is halved?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half
 Assuming no other changes, what is the effect on the rate, if the concentration of 2-bromopentane is doubled and the concentration of CH3CH2COONa is halved?
Assuming no other changes, what is the effect on the rate, if the concentration of 2-bromopentane is doubled and the concentration of CH3CH2COONa is halved?A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following SN2 reaction, 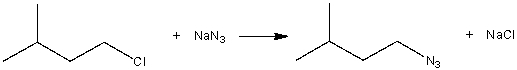 Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half
 Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the rate equation for the following reaction? ![<strong>Which of the following is the rate equation for the following reaction? </strong> A) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>] B) Rate = k[NaN<sub>3</sub>] C) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>] [NaBr] D) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>]<sup> </sup>[NaN<sub>3</sub>] E) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>]<sup>2 </sup>[NaN<sub>3</sub>]](https://storage.examlex.com/TB4454/11ea894b_ac90_45bf_bba7_8f3ec231a858_TB4454_00_TB4454_00.jpg)
A) Rate = k[CH3CH2CH2CHBrCH3]
B) Rate = k[NaN3]
C) Rate = k[CH3CH2CH2CHBrCH3] [NaBr]
D) Rate = k[CH3CH2CH2CHBrCH3] [NaN3]
E) Rate = k[CH3CH2CH2CHBrCH3]2 [NaN3]
![<strong>Which of the following is the rate equation for the following reaction? </strong> A) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>] B) Rate = k[NaN<sub>3</sub>] C) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>] [NaBr] D) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>]<sup> </sup>[NaN<sub>3</sub>] E) Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHBrCH<sub>3</sub>]<sup>2 </sup>[NaN<sub>3</sub>]](https://storage.examlex.com/TB4454/11ea894b_ac90_45bf_bba7_8f3ec231a858_TB4454_00_TB4454_00.jpg)
A) Rate = k[CH3CH2CH2CHBrCH3]
B) Rate = k[NaN3]
C) Rate = k[CH3CH2CH2CHBrCH3] [NaBr]
D) Rate = k[CH3CH2CH2CHBrCH3] [NaN3]
E) Rate = k[CH3CH2CH2CHBrCH3]2 [NaN3]

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a reasonable definition of a concerted reaction?
A) a reaction in which bond breaking occurs first
B) a reaction in which all bond-breaking and bond-forming occurs at the same time
C) a reaction in which bond forming occurs first
D) a substitution reaction
A) a reaction in which bond breaking occurs first
B) a reaction in which all bond-breaking and bond-forming occurs at the same time
C) a reaction in which bond forming occurs first
D) a substitution reaction

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name for the following compound? 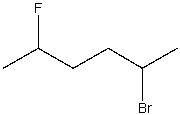


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
29
What is the correct structure for 2-bromo-3-methylbutane? 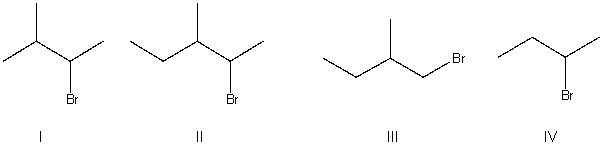
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name for the following compound? 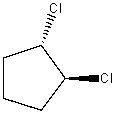
A) 1,2-bromocyclopentane
B) (1R, 2S)-1,2-dibromocyclopentane
C) (1S, 2S)-1,2-dibromocyclopentane
D) (1S, 2R)-1,2-dibromocyclopentane
E) (1R, 2R)-1,2-dibromocyclopentane

A) 1,2-bromocyclopentane
B) (1R, 2S)-1,2-dibromocyclopentane
C) (1S, 2S)-1,2-dibromocyclopentane
D) (1S, 2R)-1,2-dibromocyclopentane
E) (1R, 2R)-1,2-dibromocyclopentane

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
31
What is the IUPAC name for the following compound? 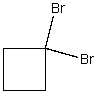


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the structure for 1-chloro-4-isopropylheptane.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
33
When drawing a curved arrow mechanism, the head of the arrow goes to__________.
A) the bond that is being formed
B) the bond that is being broken
C) the source of electrons that is being moved
D) the location to which the electrons are being moved
A) the bond that is being formed
B) the bond that is being broken
C) the source of electrons that is being moved
D) the location to which the electrons are being moved

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following SN2 reaction, 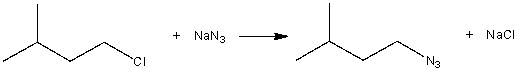 Assuming no other changes, what is the effect on the rate, if the concentration of NaN3 is tripled?
Assuming no other changes, what is the effect on the rate, if the concentration of NaN3 is tripled?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would increase the rate six times
 Assuming no other changes, what is the effect on the rate, if the concentration of NaN3 is tripled?
Assuming no other changes, what is the effect on the rate, if the concentration of NaN3 is tripled?A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would increase the rate six times

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
35
What is the correct structure for (2S,5R)-2-fluoro-5-methylnonane? 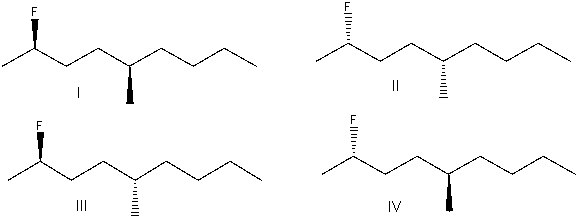
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the following SN2 reaction, 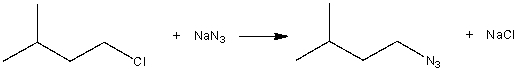 Assuming no other changes, what is the effect on the rate, if the concentration of 1-chloro-3-methylbutane is doubled?
Assuming no other changes, what is the effect on the rate, if the concentration of 1-chloro-3-methylbutane is doubled?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half
 Assuming no other changes, what is the effect on the rate, if the concentration of 1-chloro-3-methylbutane is doubled?
Assuming no other changes, what is the effect on the rate, if the concentration of 1-chloro-3-methylbutane is doubled?A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the rate equation for the following SN2 reaction? ![<strong>Which of the following is the rate equation for the following S<sub>N</sub>2 reaction? </strong> A) Rate = k[1-bromopropane] B) Rate = k[NaCN] C) Rate = k[1-bromopropane] [NaCN] D) Rate = k[1-bromopropane]<sup>2</sup> E) Rate = k[1-bromopropane]<sup>2 </sup>[NaCN]<sup>2</sup>](https://storage.examlex.com/TB4454/11ea894b_ac90_45be_bba7_8d20fe8b01be_TB4454_00_TB4454_00.jpg)
A) Rate = k[1-bromopropane]
B) Rate = k[NaCN]
C) Rate = k[1-bromopropane] [NaCN]
D) Rate = k[1-bromopropane]2
E) Rate = k[1-bromopropane]2 [NaCN]2
![<strong>Which of the following is the rate equation for the following S<sub>N</sub>2 reaction? </strong> A) Rate = k[1-bromopropane] B) Rate = k[NaCN] C) Rate = k[1-bromopropane] [NaCN] D) Rate = k[1-bromopropane]<sup>2</sup> E) Rate = k[1-bromopropane]<sup>2 </sup>[NaCN]<sup>2</sup>](https://storage.examlex.com/TB4454/11ea894b_ac90_45be_bba7_8d20fe8b01be_TB4454_00_TB4454_00.jpg)
A) Rate = k[1-bromopropane]
B) Rate = k[NaCN]
C) Rate = k[1-bromopropane] [NaCN]
D) Rate = k[1-bromopropane]2
E) Rate = k[1-bromopropane]2 [NaCN]2

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
38
Provide a curved arrow mechanism for the following SN2 reaction 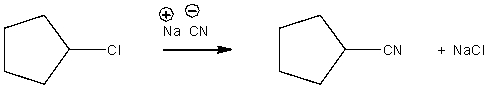


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
39
What is the correct structure for 3-ethyl-1-iodocyclohexane? 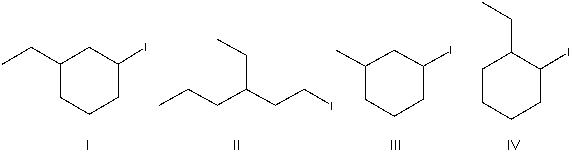
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the structure for cis-1,2-dibromocyclopentane.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
41
Predict the product for the following SN2 reaction. 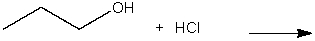
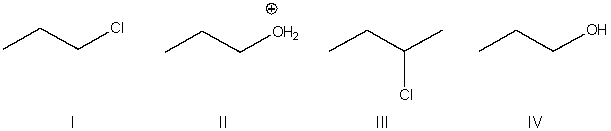
A) I
B) II
C) III
D) IV
E) None of these
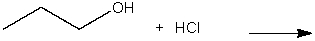

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the transition state for the following SN2 reaction. 
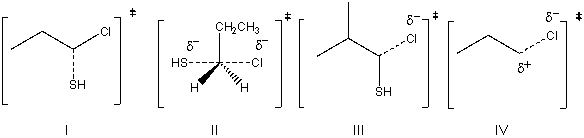
A) I
B) II
C) III
D) IV


A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following alkyl halides will undergo the fastest SN2 reaction? 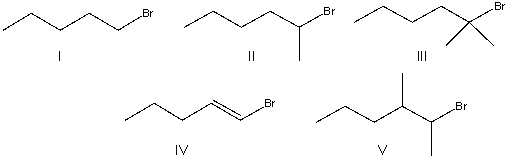
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the transition state for the following SN2 reaction. 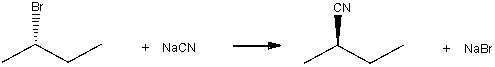
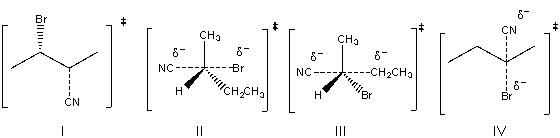
A) I
B) II
C) III
D) IV


A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
45
Predict the product for the following SN2 reaction. 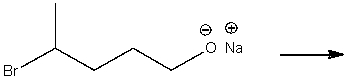
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
46
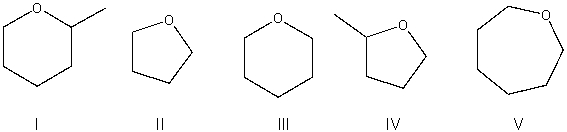
Rank the following compounds from most to least reactive in an SN2 reaction. 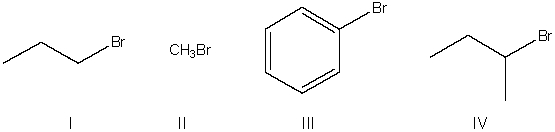
A) I > IV > II > III
B) II > I > IV > III
C) III > IV > I > II
D) IV > I > II > III
E) IV > III > I > II

A) I > IV > II > III
B) II > I > IV > III
C) III > IV > I > II
D) IV > I > II > III
E) IV > III > I > II

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is a mechanism for an SN2 reaction? 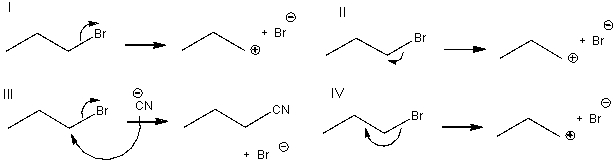
A) I
B) II
C) III
D) IV
E) Both I & II

A) I
B) II
C) III
D) IV
E) Both I & II

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
48
Provide a curved arrow mechanism and predict the product for the following SN2 reaction. 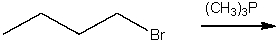


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
49
Predict the product for the following reaction.
(1R, 3S)-1-bromo-3-sec-butylcyclopentane + NaN3
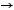
(1R, 3S)-1-bromo-3-sec-butylcyclopentane + NaN3

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the transition state for the following reaction.
(S)-1-iodo-3-methylpentane + NaSCH3
(S)-1-iodo-3-methylpentane + NaSCH3

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
51
Predict the product for the following reaction. 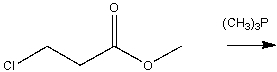
A) I
B) II
C) III
D) IV
E) None of these


A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following alkyl halides will undergo the slowest SN2 reaction? 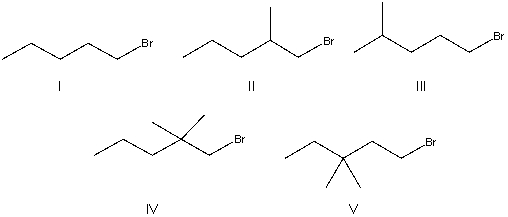
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
53
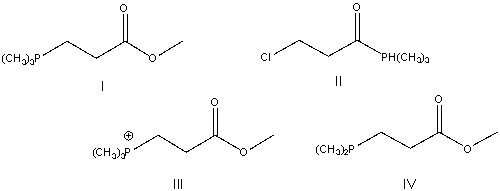
Predict the product for the following reaction. 
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the potential energy diagram for the following SN2 reaction. 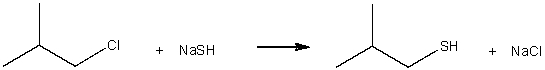
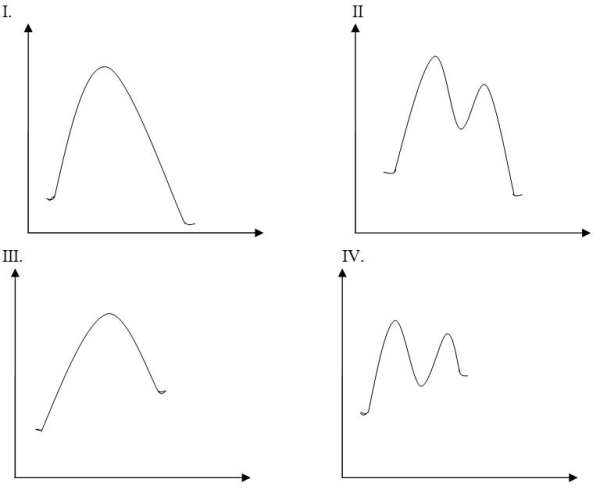
A) I
B) II
C) III
D) IV
E) None of the above


A) I
B) II
C) III
D) IV
E) None of the above

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
55
Predict the product for the following reaction. 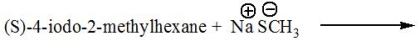
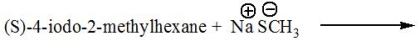

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
56
Rank the following compounds from most to least reactive in an SN2 reaction. 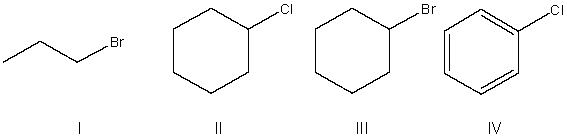
A) I > IV > II > III
B) II > I > IV > III
C) III > IV > I > II
D) I > III > II > IV
E) IV > III > I > II

A) I > IV > II > III
B) II > I > IV > III
C) III > IV > I > II
D) I > III > II > IV
E) IV > III > I > II

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following alkyl halides is essentially unreactive in an SN2 reaction? 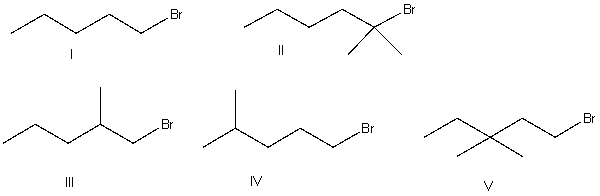
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
58
Provide a curved arrow mechanism and predict the product for the following reaction. 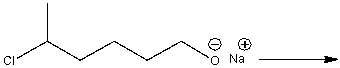


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
59
Predict the product for the following SN2 reaction. 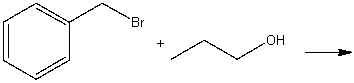
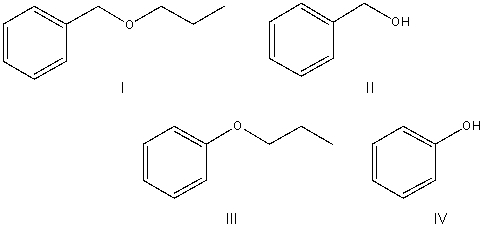
A) I
B) II
C) III
D) IV
E) None of these


A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
60
Predict the product for the following SN2 reaction. 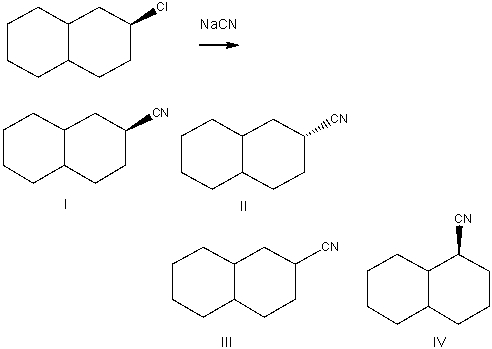
A) I
B) II
C) III
D) IV
E) Both I & II

A) I
B) II
C) III
D) IV
E) Both I & II

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
61
Predict the product for the following SN2 reaction. 
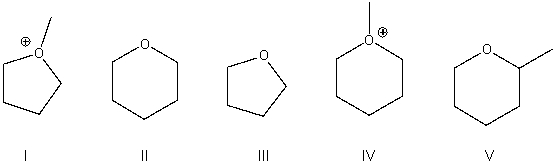
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
62
Identify the IUPAC name for the following compound. 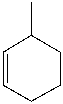
A) 1-methyl-2-cyclohexene
B) 2-methylcyclohexene
C) 3-methylcyclohexene
D) 1-methyl-5-cyclohexene

A) 1-methyl-2-cyclohexene
B) 2-methylcyclohexene
C) 3-methylcyclohexene
D) 1-methyl-5-cyclohexene

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the methylating agent used in biological systems.
A) methyl iodide
B) S-adenosylmethionine
C) methylalanine
D) methylproline
E) methyl bromide
A) methyl iodide
B) S-adenosylmethionine
C) methylalanine
D) methylproline
E) methyl bromide

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is a protic solvent? 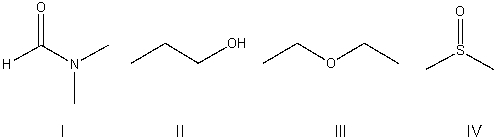
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
65
Describe a strong nucleophile.
A) an anion
B) a cation
C) a radical
D) a neutral compound
A) an anion
B) a cation
C) a radical
D) a neutral compound

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is NOT a nucleophile?
A) OH-
B) NH3
C) CH3OH
D) NH4+
E) All of these
A) OH-
B) NH3
C) CH3OH
D) NH4+
E) All of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is a strong nucleophile?
A) OH-
B) H2O
C) CH3OH
D) NH4+
E) All of these
A) OH-
B) H2O
C) CH3OH
D) NH4+
E) All of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is a weak nucleophile?
A) OH-
B) H2O
C) CH3O-
D) NH4+
E) All of these
A) OH-
B) H2O
C) CH3O-
D) NH4+
E) All of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
69
Identify which of the following that is NOT an effect of adrenaline.
A) increases heart rate
B) elevates sugar level
C) provides boost of energy
D) increases level of oxygen
E) makes one sleepy
A) increases heart rate
B) elevates sugar level
C) provides boost of energy
D) increases level of oxygen
E) makes one sleepy

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
70
Provide an IUPAC name for the following compound. 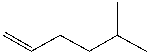


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is the IUPAC name for the following compound? 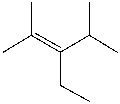
A) 2-ethyl-1,1,3-trimethylbutene
B) 3-ethyl-2,4-dimethyl-2-pentene
C) 2,4-dimethylhexene
D) 4-ethyl-1,3-dimethyl-3-pentene

A) 2-ethyl-1,1,3-trimethylbutene
B) 3-ethyl-2,4-dimethyl-2-pentene
C) 2,4-dimethylhexene
D) 4-ethyl-1,3-dimethyl-3-pentene

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
72
Predict the product for the following SN2 reaction. 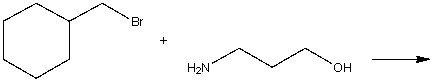


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following shows a mechanism of a concerted elimination? 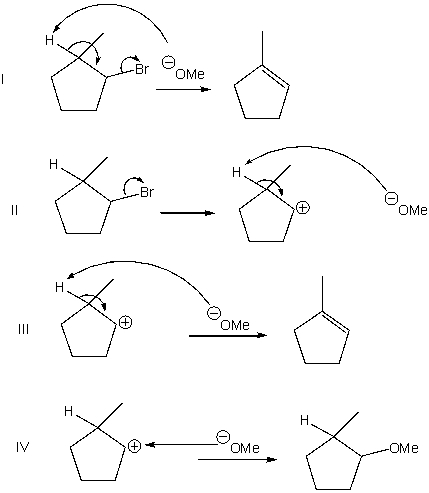
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the SN2 product and provide a curved arrow mechanism for the formation of the product. 


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
75
What set of reaction conditions would favor an SN2 reaction on 2-bromo-3-methylbutane?
A) weak nucleophile in a protic solvent
B) weak nucleophile in an aprotic solvent
C) strong nucleophile in a protic solvent
D) strong nucleophile in an aprotic solvent
A) weak nucleophile in a protic solvent
B) weak nucleophile in an aprotic solvent
C) strong nucleophile in a protic solvent
D) strong nucleophile in an aprotic solvent

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is an aprotic solvent? 
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
77
Identify the type of elimination in an E2 mechanism.
A) alpha elimination
B) beta elimination
C) gamma elimination
D) delta elimination
E) omega elimination
A) alpha elimination
B) beta elimination
C) gamma elimination
D) delta elimination
E) omega elimination

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the structure of 2,3-dimethyl-1-pentene.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following describes the difference between protic and aprotic solvents?
A) protic solvents stabilize anions only
B) aprotic solvents stabilize anions only
C) protic solvents stabilize cations only
D) aprotic solvents stabilize both cations and anions
E) protic solvents stabilize both cations and anions
A) protic solvents stabilize anions only
B) aprotic solvents stabilize anions only
C) protic solvents stabilize cations only
D) aprotic solvents stabilize both cations and anions
E) protic solvents stabilize both cations and anions

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
80
Provide a curved arrow mechanism for the following SN2 reaction. 


Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck


