Deck 17: Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 17: Aromatic Compounds
1
What is the IUPAC name for the following compound? 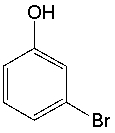
A) 5-bromophenol
B) 3-bromophenol
C) 5-bromoaniline
D) 3-bromoaniline
E) 1-bromophenol

A) 5-bromophenol
B) 3-bromophenol
C) 5-bromoaniline
D) 3-bromoaniline
E) 1-bromophenol
3-bromophenol
2
Give the common name for o-xylene.
A) hydroxybenzene
B) aminobenzene
C) 1,2-dimethylbenzene
D) ethylbenzene
E) 1,3-dimethylbenzene
A) hydroxybenzene
B) aminobenzene
C) 1,2-dimethylbenzene
D) ethylbenzene
E) 1,3-dimethylbenzene
1,2-dimethylbenzene
3
Which of the following is another name for 4-chlorobenzaldehyde?
A) o-chlorobenzaldehyde
B) m-chlorobenzaldehyde
C) p-chlorobenzaldehyde
D) styrene
E) 1-chlorobenzaldehyde
A) o-chlorobenzaldehyde
B) m-chlorobenzaldehyde
C) p-chlorobenzaldehyde
D) styrene
E) 1-chlorobenzaldehyde
p-chlorobenzaldehyde
4
Identify the functional group in acetophenone.
A) ether
B) alkene
C) carboxylic acid
D) aldehyde
E) ketone
A) ether
B) alkene
C) carboxylic acid
D) aldehyde
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
What is the correct structure for 4-amino-2-chlorophenol? 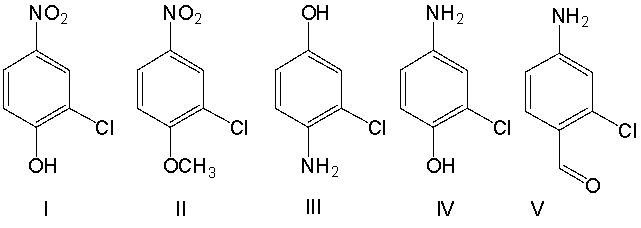
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name for the following compound? 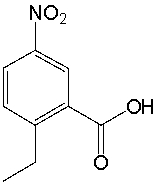
A) 6-ethyl-3-nitrobenzoic acid
B) 1-ethyl-4-nitrobenzoic acid
C) 2-ethyl-5-nitrobenzoic acid
D) 2-ethyl-5-nitrobenzaldehyde
E) 4-nitro-3-carboxyethylbenzene

A) 6-ethyl-3-nitrobenzoic acid
B) 1-ethyl-4-nitrobenzoic acid
C) 2-ethyl-5-nitrobenzoic acid
D) 2-ethyl-5-nitrobenzaldehyde
E) 4-nitro-3-carboxyethylbenzene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
What is the IUPAC name for the following compound? 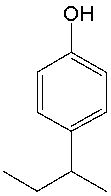
A) 4-butylphenol
B) 1-sec-butylphenol
C) 4-propylphenol
D) 4-sec-butylphenol
E) 1-isobutylphenol

A) 4-butylphenol
B) 1-sec-butylphenol
C) 4-propylphenol
D) 4-sec-butylphenol
E) 1-isobutylphenol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Give the common name for anisole.
A) hydroxybenzene
B) aminobenzene
C) methylbenzene
D) ethylbenzene
E) methoxybenzene
A) hydroxybenzene
B) aminobenzene
C) methylbenzene
D) ethylbenzene
E) methoxybenzene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
What is the structure for 1,3-diphenylbutane?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
When the benzene ring is treated as a substituent, it is called a _________ group

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
For disubstituted benzene derivatives, the descriptors ortho, meta, para are used when the parent is usually a ________.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Give the common name for phenol.
A) hydroxybenzene
B) aminobenzene
C) methylbenzene
D) ethylbenzene
E) methoxybenzene
A) hydroxybenzene
B) aminobenzene
C) methylbenzene
D) ethylbenzene
E) methoxybenzene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
What is the correct order of the names for the following compound? 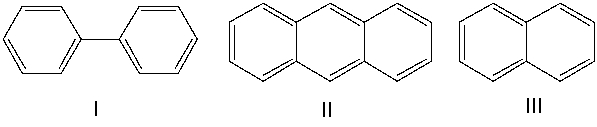
A) I-naphthalene; II-triphenyl; III-biphenyl
B) I-naphthalene; II-phenanthrene; III-biphenyl
C) I-biphenyl; II-anthracene; III-naphthalene
D) I-naphthalene; II-anthracene; III-biphenyl
E) I-biphenyl; II- phenanthrene; III-naphthalene

A) I-naphthalene; II-triphenyl; III-biphenyl
B) I-naphthalene; II-phenanthrene; III-biphenyl
C) I-biphenyl; II-anthracene; III-naphthalene
D) I-naphthalene; II-anthracene; III-biphenyl
E) I-biphenyl; II- phenanthrene; III-naphthalene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the functional group in styrene.
A) ether
B) alkene
C) carboxylic acid
D) aldehyde
E) ketone
A) ether
B) alkene
C) carboxylic acid
D) aldehyde
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
What is the common name for the following compound? 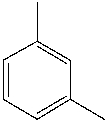


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name for the following compound? 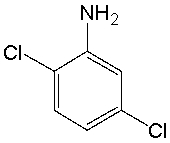
A) 3,4-dichloroaniline
B) 2,4-dichloroaniline
C) 2,5-dichloroaniline
D) 3,6-dichloroaniline
E) 2,6-dichloroaniline

A) 3,4-dichloroaniline
B) 2,4-dichloroaniline
C) 2,5-dichloroaniline
D) 3,6-dichloroaniline
E) 2,6-dichloroaniline

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
What is the structure for p-aminobenzoic acid (PABA)?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
What is the structure for 4-amino-2-bromophenol? 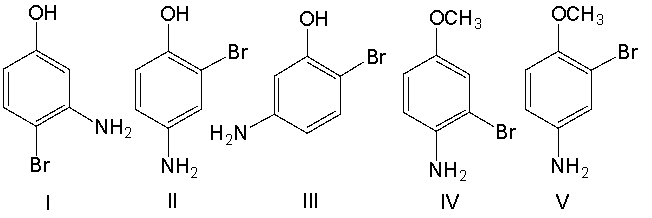
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
Give the common name for toluene.
A) hydroxybenzene
B) aminobenzene
C) methylbenzene
D) ethylbenzene
E) methoxybenzene
A) hydroxybenzene
B) aminobenzene
C) methylbenzene
D) ethylbenzene
E) methoxybenzene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name for the following compound? 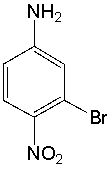
A) 5-bromo-4-nitroaniline
B) 5-bromo-p-nitroaniline
C) 1-bromo-2-nitroaniline
D) 3-bromo-4-nitroaniline
E) p-nitro-m-bromoaniline

A) 5-bromo-4-nitroaniline
B) 5-bromo-p-nitroaniline
C) 1-bromo-2-nitroaniline
D) 3-bromo-4-nitroaniline
E) p-nitro-m-bromoaniline

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
Using a Frost circle, draw the molecular orbital energy diagram for the cyclopropenyl anion and predict if it is aromatic. 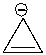
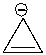

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
For a compound to be aromatic, it must have a planar cyclic conjugated system along with an _____ number of electron pairs.
A) even
B) odd
C) either even or odd
D) none of these
A) even
B) odd
C) either even or odd
D) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name for the following compound? 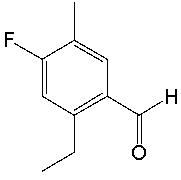


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
What is the structure for styrene?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
The carbon-carbon bonds in benzene are _________.
A) of equal length and are shorter than the double bond
B) of equal length and are midway between a single bond and a double bond
C) of equal length and are longer than the single bond
D) of unequal length and alternate as single and double bonds
E) none of these
A) of equal length and are shorter than the double bond
B) of equal length and are midway between a single bond and a double bond
C) of equal length and are longer than the single bond
D) of unequal length and alternate as single and double bonds
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following describes the current view of the two Kekulé structures for benzene?
A) they are both correct structures for benzene
B) the two structures are in equilibrium
C) the two structures adequately describe benzene
D) benzene is a resonance hybrid of the two structures
E) None of the above
A) they are both correct structures for benzene
B) the two structures are in equilibrium
C) the two structures adequately describe benzene
D) benzene is a resonance hybrid of the two structures
E) None of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Which one of the following statements is not true for a compound to be considered as aromatic?
A) The compound must be cyclic and planar
B) The compound must be monocyclic.
C) The compound must have a conjugated system with a p orbital at every vertex
D) The compound must satisfy Hückel's rule -must have (4n + 2) electrons.
E) none of these
A) The compound must be cyclic and planar
B) The compound must be monocyclic.
C) The compound must have a conjugated system with a p orbital at every vertex
D) The compound must satisfy Hückel's rule -must have (4n + 2) electrons.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
The difference between the amount of heat actually released upon the hydrogenation of benzene and that calculated for the hydrogenation of an imaginary cyclohexatriene is called the ____ of benzene.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
According to molecular orbital theory, how many non-bonding molecular orbitals does benzene have?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name for the following compound? 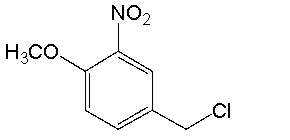


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
According to molecular orbital theory, how many -bonding molecular orbitals does benzene have?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the number of electrons present in an aromatic compound.
A) 4n + 2
B) 2n + 2
C) 4n
D) 2n
E) 3n
A) 4n + 2
B) 2n + 2
C) 4n
D) 2n
E) 3n

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
According to molecular orbital theory, how many -antibonding molecular orbitals does benzene have?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
What is the structure for 3-isobutyl-5-isopropylaniline?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
What is the main difference between an aromatic and antiaromatic compound?
A) Aromatic compounds must be cyclic and planar, but not antiaromatic compounds
B) Aromatic compounds must be monocyclic.
C) Antiaromatic compounds must have a conjugated system with a p orbital at every vertex
D) Aromatic compounds must satisfy Hückel's rule.
E) none of these
A) Aromatic compounds must be cyclic and planar, but not antiaromatic compounds
B) Aromatic compounds must be monocyclic.
C) Antiaromatic compounds must have a conjugated system with a p orbital at every vertex
D) Aromatic compounds must satisfy Hückel's rule.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the number of electrons present in an antiaromatic compound.
A) 4n + 2
B) 2n + 2
C) 4n
D) none
E) none of these
A) 4n + 2
B) 2n + 2
C) 4n
D) none
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name for the following compound? 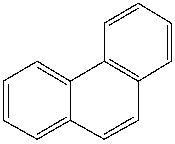


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Molecular orbitals of equal energy are referred to as _________ orbitals.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
According to the molecular orbital theory how many molecular orbitals are formed when the six p-orbitals of benzene combine?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the scientist credited with proposing the structure of benzene.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Identify the structure that is an annulene. 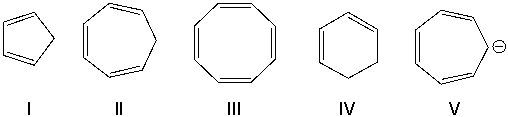
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of the following compounds is aromatic? 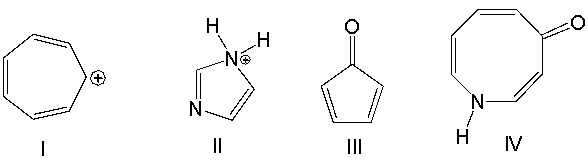
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Using a Frost circle, draw the molecular orbital energy diagram for the cyclononatetraenyl cation and predict if it is aromatic. 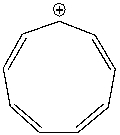


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Which one of the following compounds is antiaromatic? 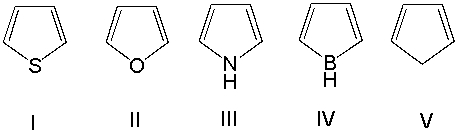
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following compounds is nonaromatic? 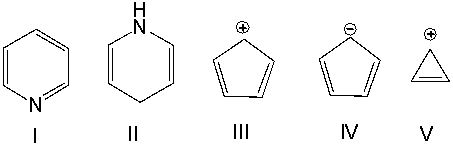
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Using a Frost circle, draw the molecular orbital energy diagram for the tropylium cation and predict if it is aromatic. 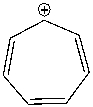


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Which one of the following compounds is aromatic? 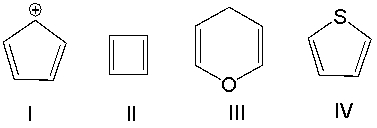
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the following compounds is antiaromatic? 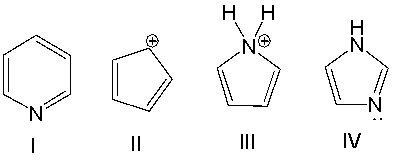
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 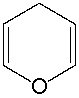


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following compounds is nonaromatic? 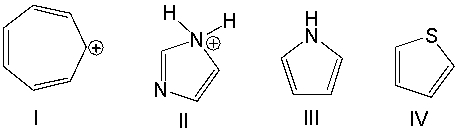
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 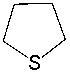


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 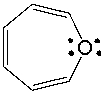


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the structure that is not an annulene. 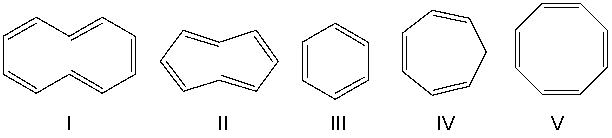
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 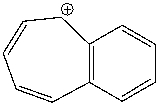


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following compounds is antiaromatic? 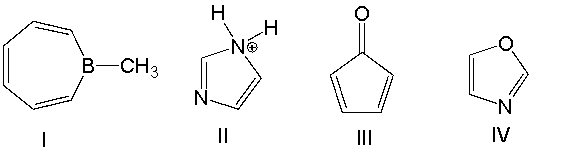
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 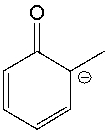


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Using a Frost circle, draw the molecular orbital energy diagram for the cyclopentadienyl anion and predict if it is aromatic. 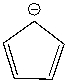


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
Which one of the following compounds is aromatic? 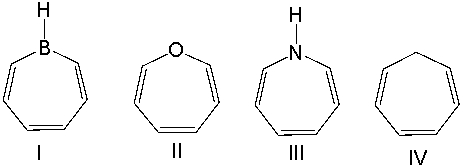
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following compounds is aromatic? 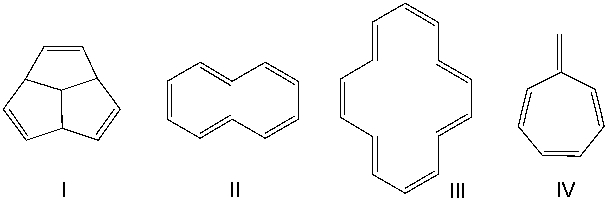
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following annulenes is not aromatic?
A) [6]-annulene
B) [14]-annulene
C) [16]-annulene
D) [18]-annulene
E) [22]-annulene
A) [6]-annulene
B) [14]-annulene
C) [16]-annulene
D) [18]-annulene
E) [22]-annulene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Identify the aromatic hydrocarbon with the highest stabilization energy per ring.
A) phenanthrene
B) benzene
C) naphthalene
D) anthracene
A) phenanthrene
B) benzene
C) naphthalene
D) anthracene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
Which one of the following compounds will undergo the fastest SN1 reaction? Explain your choice. 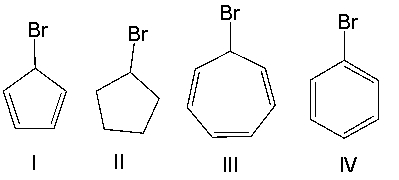
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 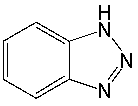


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Provide the product for the following reaction. 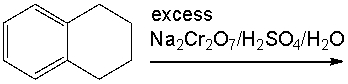
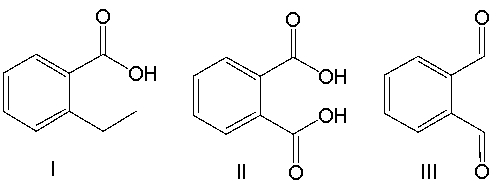
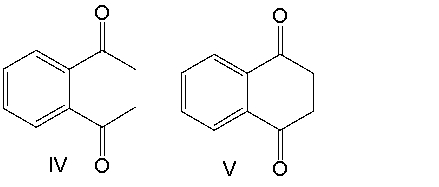
A) I
B) II
C) III
D) IV
E) V
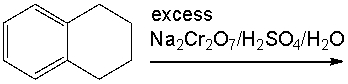


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the reagent(s) necessary to convert toluene to benzoic acid.
A) Na2Cr2O7/H2SO4/H2O
B) 1. NBS, 2.NaOH
C) 1. LiAlH4 2. H3O+
D) H2, Pd
E) 1. CO2, 2. H3O+
A) Na2Cr2O7/H2SO4/H2O
B) 1. NBS, 2.NaOH
C) 1. LiAlH4 2. H3O+
D) H2, Pd
E) 1. CO2, 2. H3O+

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 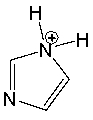


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the product for the following reaction. Explain your answer. 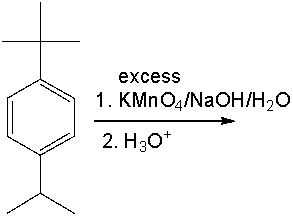


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Both pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, whereas pyrrole is unreactive. Provide an explanation for this observed reactivity. 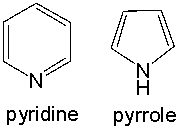


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
Which one of the following compounds is most acidic? Explain your choice. 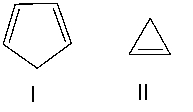


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
Which one of the following compounds will undergo the fastest SN1 reaction? Explain your choice. 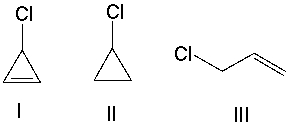
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following compounds is most acidic? 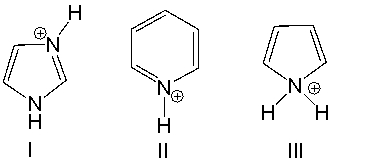
A) I
B) II
C) III
D) both I and II
E) both II and III

A) I
B) II
C) III
D) both I and II
E) both II and III

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
Classify the following compounds as aromatic, antiaromatic, or nonaromatic. Explain your choice. 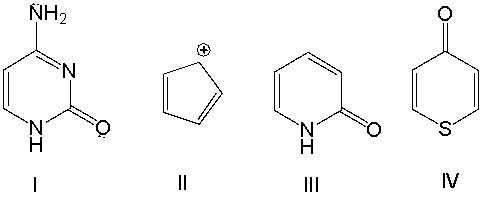


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Which one of the following compounds is most acidic? 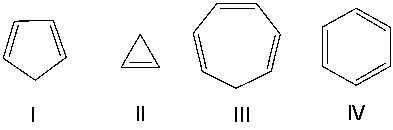
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Identify the aromatic hydrocarbon with the highest stabilization energy.
A) phenanthrene
B) benzene
C) naphthalene
D) anthracene
A) phenanthrene
B) benzene
C) naphthalene
D) anthracene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Classify the following compounds as aromatic, antiaromatic, or nonaromatic. Explain your choice. 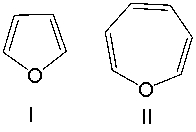


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
Provide the structure of the major product(s) for the following reaction. 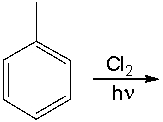
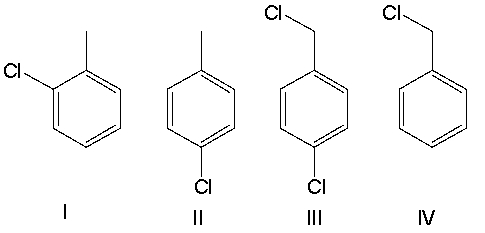
A) I
B) II
C) III
D) IV
E) Both I and II


A) I
B) II
C) III
D) IV
E) Both I and II

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
The pKa of cyclopentadiene (I) and cycloheptatriene (II) is around 16 and 36 respectively. Explain the difference in the two pKa values. 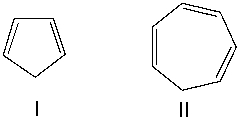


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Which one of the following compounds is most acidic? 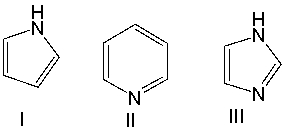
A) I
B) II
C) III
D) I & III
E) II & III

A) I
B) II
C) III
D) I & III
E) II & III

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Classify the following compound as aromatic, antiaromatic, or nonaromatic. Explain your choice. 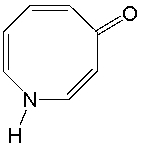


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
Provide the product for the following reaction. 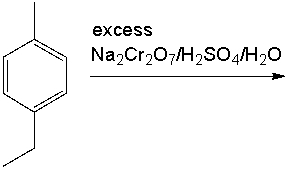
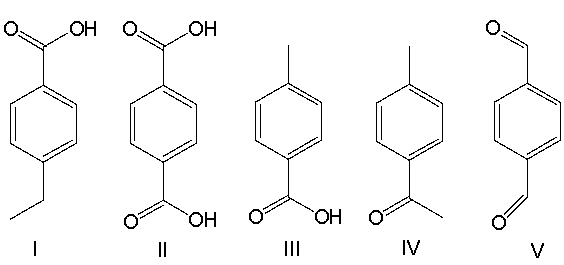
A) I
B) II
C) III
D) IV
E) V
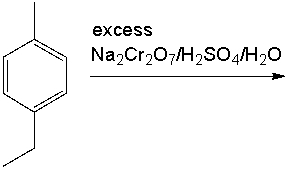

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


