Deck 16: Conjugated Pi Systems and Pericyclic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 16: Conjugated Pi Systems and Pericyclic Reactions
1
Provide the structure for (1E,3Z)-1-methoxy-2-methyl-1,3-pentadiene. 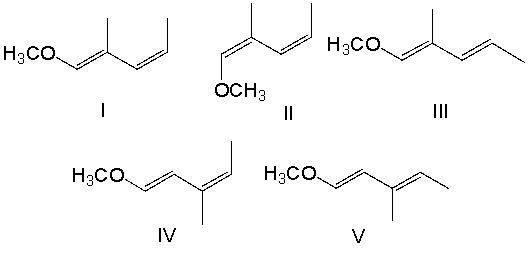
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
I
2
What is the IUPAC name for the following compound? 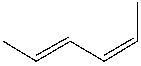
A) (2E, 4Z)-2,4-hexadiene
B) (2E, 4Z)-1,4-dimethyl-1,3-butadiene
C) (2Z, 4Z)-1,4-dimethyl-1,3-butadiene
D) (2Z, 4Z)-2,4-hexadiene
E) None of these

A) (2E, 4Z)-2,4-hexadiene
B) (2E, 4Z)-1,4-dimethyl-1,3-butadiene
C) (2Z, 4Z)-1,4-dimethyl-1,3-butadiene
D) (2Z, 4Z)-2,4-hexadiene
E) None of these
(2E, 4Z)-2,4-hexadiene
3
What is the IUPAC name for the following compound? 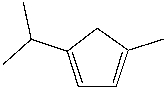

1-isopropyl-4-methyl-1,3-cyclopentadiene
4
Provide the structure for (Z)-2-methyl-2,4-hexadiene.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name for the following compound? 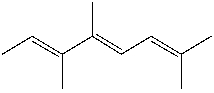
A) (2E,4Z,6E)-3,4,7,8-tetramethyl-2,4,6-heptatriene
B) (2Z,4E)-3,4,7-trimethyl-2,4,6-octatriene
C) (2E,4Z,6E)-2,5,6,7-tetramethyl-3,5,7-heptatriene
D) (2E,4Z)- 2,5,6-trimethyl-3,5,7-octatriene
E) (4E,6E)-2,5,6-trimethyl-2,4,6-octatriene

A) (2E,4Z,6E)-3,4,7,8-tetramethyl-2,4,6-heptatriene
B) (2Z,4E)-3,4,7-trimethyl-2,4,6-octatriene
C) (2E,4Z,6E)-2,5,6,7-tetramethyl-3,5,7-heptatriene
D) (2E,4Z)- 2,5,6-trimethyl-3,5,7-octatriene
E) (4E,6E)-2,5,6-trimethyl-2,4,6-octatriene

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is an isolated diene? 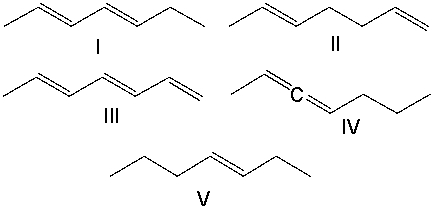
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
Classify the following compounds as having cumulated, conjugated or isolated double bonds? 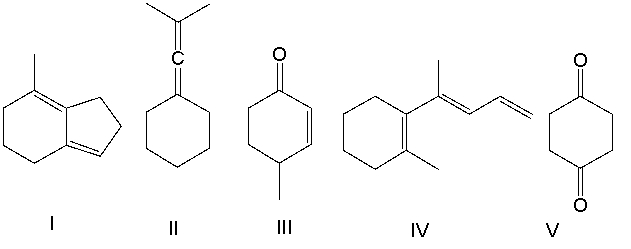


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
Zingiberene, a terpene found in ginger, has the following structure. Classify the bonds in zingiberene as conjugated, cumulated or isolated. 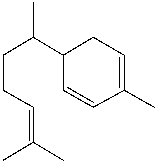


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the cumulated diene.
A) 4-methyl-1,3-heptadiene
B) 3-methyl-1,5-heptadiene
C) 2-methyl-2,4-heptadiene
D) 4-methyl-1,4-heptadiene
E) 5-methyl-2,3-heptadiene
A) 4-methyl-1,3-heptadiene
B) 3-methyl-1,5-heptadiene
C) 2-methyl-2,4-heptadiene
D) 4-methyl-1,4-heptadiene
E) 5-methyl-2,3-heptadiene

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
Dienes with adjacent π bonds are classified as ______.
A) isolated
B) cumulated
C) skipped
D) conjugated
E) none of these
A) isolated
B) cumulated
C) skipped
D) conjugated
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds have isolated double bonds? 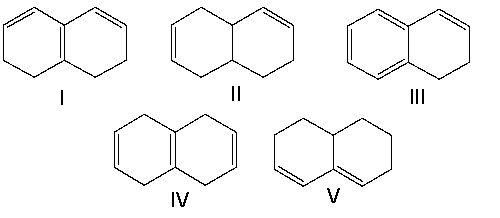
A) II and IV
B) III and V
C) I, III, and V
D) I and V
E) I and III

A) II and IV
B) III and V
C) I, III, and V
D) I and V
E) I and III

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name for the following compound? 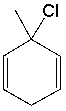
A) 1-chloro-1-methyl-2,5-cyclohexadiene
B) 3-chloro-3-methyl-1,4-cyclohexadiene
C) 6-chloro-6-methyl-1,4-cyclohexadiene
D) 2-chloro-2-methyl-1,3-cyclohexadiene
E) None of these

A) 1-chloro-1-methyl-2,5-cyclohexadiene
B) 3-chloro-3-methyl-1,4-cyclohexadiene
C) 6-chloro-6-methyl-1,4-cyclohexadiene
D) 2-chloro-2-methyl-1,3-cyclohexadiene
E) None of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
Vitamin D3 has the following structure. Classify the bonds in vitamin D3 as conjugated, cumulated or isolated. 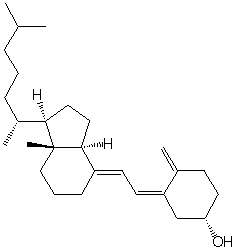


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the conjugated diene(s). 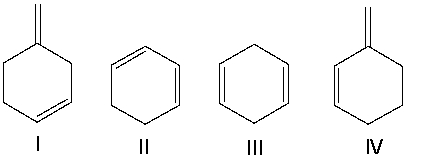
A) I
B) II
C) III
D) IV
E) II & IV

A) I
B) II
C) III
D) IV
E) II & IV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the isolated dienes.
A) 4-methyl-1,3-heptadiene
B) 3-methyl-1,5-heptadiene
C) 2-methyl-2,4-heptadiene
D) 4-methyl-1,4-heptadiene
E) 5-methyl-2,3-heptadiene
A) 4-methyl-1,3-heptadiene
B) 3-methyl-1,5-heptadiene
C) 2-methyl-2,4-heptadiene
D) 4-methyl-1,4-heptadiene
E) 5-methyl-2,3-heptadiene

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
Identify the conjugated diene(s).
A. 4-methyl-1,3-heptadiene
B. 3-methyl-1,5-heptadiene
C. 2-methyl-2,4-heptadiene
D. 4-methyl-1,4-heptadiene
E. 5-methyl-2,3-heptadiene
A. 4-methyl-1,3-heptadiene
B. 3-methyl-1,5-heptadiene
C. 2-methyl-2,4-heptadiene
D. 4-methyl-1,4-heptadiene
E. 5-methyl-2,3-heptadiene

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
Provide the structure for (E)-1,3-pentadiene.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
Dienes with alternate π and σ bonds are classified as ______.
A) isolated
B) cumulated
C) skipped
D) conjugated
E) none of these
A) isolated
B) cumulated
C) skipped
D) conjugated
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds have conjugated double bonds? 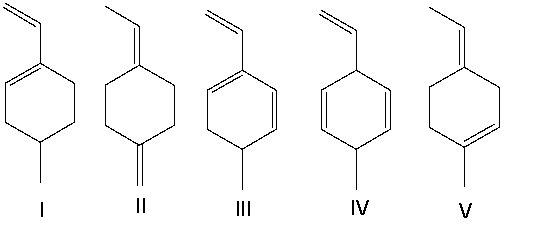
A) II and V
B) II, IV, and V
C) I and III
D) I, III, and IV
E) all of them

A) II and V
B) II, IV, and V
C) I and III
D) I, III, and IV
E) all of them

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
Dienes with bonds separated by two or more σ bonds are classified as ______.
A) isolated
B) cumulated
C) skipped
D) conjugated
E) none of these
A) isolated
B) cumulated
C) skipped
D) conjugated
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
Rank the following dienes in order of decreasing heat of hydrogenation (highest to lowest). 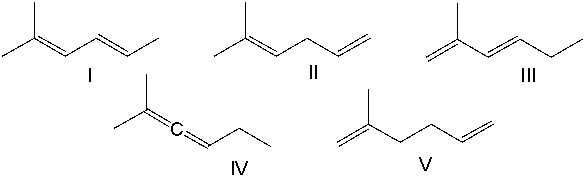


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the product(s) for the following reaction. 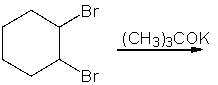
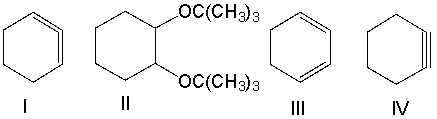
A) I
B) II
C) III
D) IV
E) both I & III


A) I
B) II
C) III
D) IV
E) both I & III

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following indicated C-C bonds is(are) the shortest? 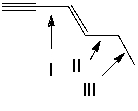
A) I
B) II
C) III
D) both I and II

A) I
B) II
C) III
D) both I and II

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following dienes is most stable?
A) CH3CH=CHCH=CHCH3
B) CH3CH=CHCH2CH=CH2
C) CH2=CHCH2CH2CH=CH2
D) CH2=CHCH(CH3)CH=CH2
E) CH3CH=C=CHCH2CH3
A) CH3CH=CHCH=CHCH3
B) CH3CH=CHCH2CH=CH2
C) CH2=CHCH2CH2CH=CH2
D) CH2=CHCH(CH3)CH=CH2
E) CH3CH=C=CHCH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the product for the following reaction. 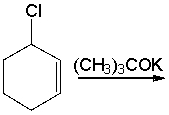


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
Provide the reagents necessary to carry out the following conversion. 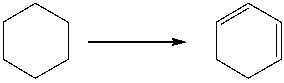


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
Rank the following dienes in order of decreasing heat of hydrogenation (highest to lowest). 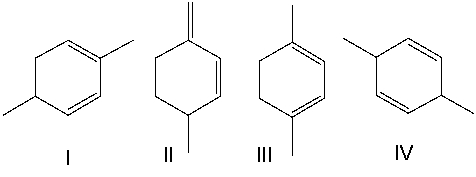


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
Both s-cis and s-trans conformers of 1,3-butadiene have a continuous conjugated system. Which of the following statements is true about the s-cis conformer?
A) The s-cis conformer is lower in energy than the s-trans conformer
B) The s-cis conformer is higher in energy than the s-trans conformer
C) The s-cis conformer has equal energy as the s-trans conformer
D) none of these
A) The s-cis conformer is lower in energy than the s-trans conformer
B) The s-cis conformer is higher in energy than the s-trans conformer
C) The s-cis conformer has equal energy as the s-trans conformer
D) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
Rank the following dienes in order of increasing stability (least to most). 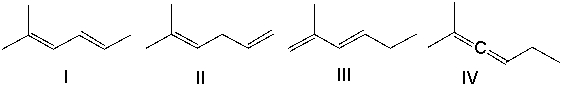
A) I < IV < III < II
B) III < II < I < IV
C) IV < II < III < I
D) II < IV < III < I
E) IV < III < II < I

A) I < IV < III < II
B) III < II < I < IV
C) IV < II < III < I
D) II < IV < III < I
E) IV < III < II < I

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
Provide the reagents necessary to carry out the following conversion. 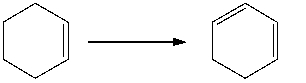


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
Predict the product(s) for the following reaction. 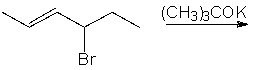
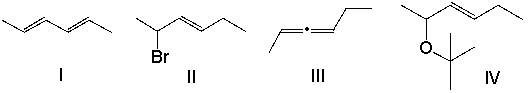
A) I
B) II
C) III
D) IV
E) both I & III


A) I
B) II
C) III
D) IV
E) both I & III

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following dienes is least stable? 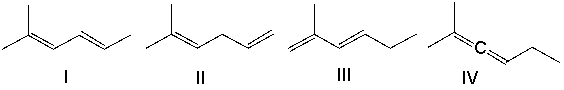
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
Predict the product for the following reaction. 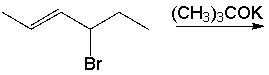


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following dienes is least stable?
A) CH3CH=CHCH=CHCH3
B) CH3CH=CHCH2CH=CH2
C) CH2=CHCH2CH2CH=CH2
D) CH2=CHCH(CH3)CH=CH2
E) CH3CH=C=CHCH2CH3
A) CH3CH=CHCH=CHCH3
B) CH3CH=CHCH2CH=CH2
C) CH2=CHCH2CH2CH=CH2
D) CH2=CHCH(CH3)CH=CH2
E) CH3CH=C=CHCH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
Provide the structure for (S,E)-3-t-butyl-4-methyl-1,4-hexadiene.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
36
Which one of the following dienes will have the highest heat of hydrogenation? 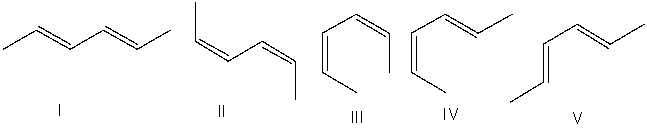
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following dienes will have the lowest heat of hydrogenation? 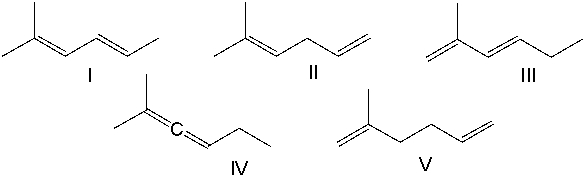
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following dienes is most stable? 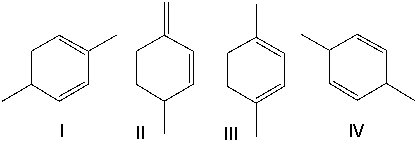
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
Provide the reagents necessary to carry out the following conversion. 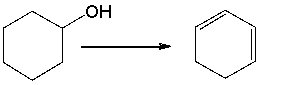


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following indicated C-C bonds is (are) the longest? 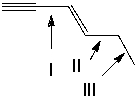
A) I
B) II
C) III
D) both I and II

A) I
B) II
C) III
D) both I and II

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
The reaction of 1,3-butadiene with hydrogen halide at 0°C will undergo _____ -addition under ____________ control.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
The reaction of 1,3-butadiene with hydrogen halide at 40°C will undergo _____ -addition under ____________ control.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following represents the lowest energy -bonding molecular orbital of 1,3-butadiene? 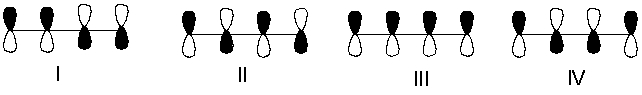
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the product of a thermodynamically-controlled reaction.
A) the most stable product
B) the product whose formation requires the smallest free energy of activation
C) the product that can be formed in the fewest steps
D) the product that is formed at the fastest rate
E) none of these
A) the most stable product
B) the product whose formation requires the smallest free energy of activation
C) the product that can be formed in the fewest steps
D) the product that is formed at the fastest rate
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
Predict the major product for the following reaction. 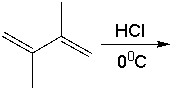


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
Provide the structure of the 1,4 addition product for the reaction of 1,3-hexadiene with Br2/CCl4.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
Provide the structure of the 1,2 addition product for the following reaction. 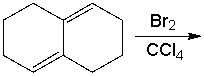


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
Predict the possible major products for the following reaction. 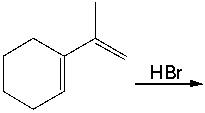


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
How many -bonding molecular orbitals does 1,3-pentadiene have?
A) 1
B) 2
C) 3
D) 4
E) none
A) 1
B) 2
C) 3
D) 4
E) none

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
Which major product(s) is(are) formed in the following reaction? 
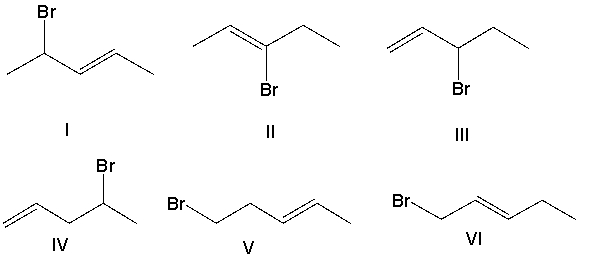
A) I and V
B) II and IV
C) III and IV
D) I, III and VI
E) II, IV and V


A) I and V
B) II and IV
C) III and IV
D) I, III and VI
E) II, IV and V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the products of a reaction under kinetic control.
A) the most stable product
B) the product whose formation requires the smallest free energy of activation
C) the product that can be formed in the fewest steps
D) the product that is formed at the fastest rate
E) none of these
A) the most stable product
B) the product whose formation requires the smallest free energy of activation
C) the product that can be formed in the fewest steps
D) the product that is formed at the fastest rate
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
Which one of the following represents the highest energy -antibonding molecular orbital of 1,3-butadiene? 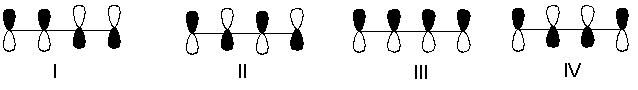
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
Which one of the following represents the LUMO of 1,3,5-hexatriene? 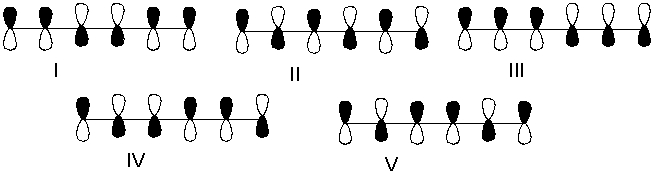
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
How many electrons does the LUMO of 2,4-hexadiene have in its ground state?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following represents the HOMO of 1,3,5-hexatriene? 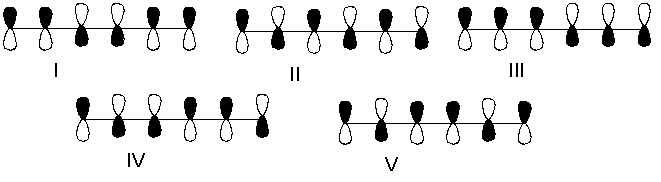
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
How many electrons does the HOMO of 2,4-hexadiene have in its ground state?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
How many electrons does the HOMO of 1,3-pentadiene have in its excited state?
A) 1
B) 2
C) 3
D) 4
E) none
A) 1
B) 2
C) 3
D) 4
E) none

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
Which one of the following compounds is not a product of the reaction between 1,3-butadiene and HBr?
A) (S)-3-bromo-1-butene
B) (R)-3-bromo-1-butene
C) (E)-1-bromo-2-butene
D) (Z)-1-bromo-2-butene
E) (Z)-2-bromo-2-butene
A) (S)-3-bromo-1-butene
B) (R)-3-bromo-1-butene
C) (E)-1-bromo-2-butene
D) (Z)-1-bromo-2-butene
E) (Z)-2-bromo-2-butene

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
Predict the possible products for the following reaction and provide a curved arrow mechanism for the formation of these products. 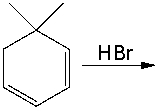


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
Predict the product(s) for the following reaction. 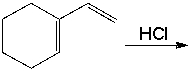


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
Predict the major product for the following reaction. 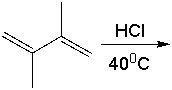


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following diene(s) cannot undergo the Diels-Alder reaction? 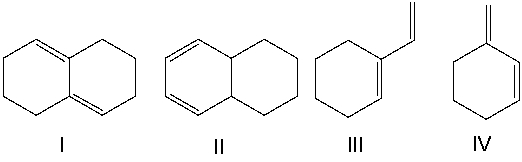
A) I
B) II
C) III
D) IV
E) I & IV

A) I
B) II
C) III
D) IV
E) I & IV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the pericyclic reaction in which two sigma bonds are broken and two pi bonds are formed.
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the pericyclic reaction in which one sigma bond is formed and one pi bond is broken.
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
Which one of the following dienophiles is least reactive in the Diels-Alder reaction? 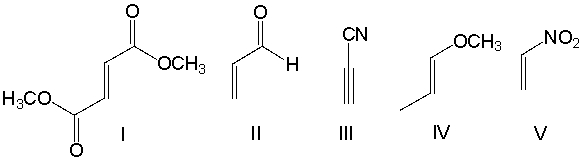
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
The polymerization of isoprene to produce synthetic rubber is a special case of ____- addition.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
Identify the pericyclic reaction in which no sigma bonds are formed and no pi bonds are broken.
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following diene(s) can undergo the Diels-Alder reaction? 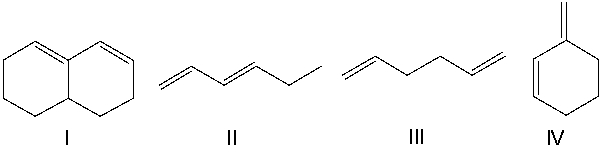
A) I
B) II
C) III
D) IV
E) All of the these

A) I
B) II
C) III
D) IV
E) All of the these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
What is the correct classification of the following pericyclic reaction? 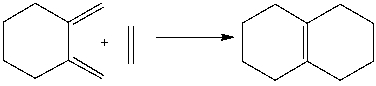
A) electrophilic addition
B) sigmatropic rearrangement
C) cycloaddition
D) electrocyclic reaction
E) C & D

A) electrophilic addition
B) sigmatropic rearrangement
C) cycloaddition
D) electrocyclic reaction
E) C & D

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
Predict the major product for the following reaction and explain why it is major product. 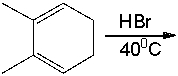


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
Predict the major product for the following reaction. 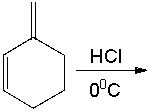


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
Which statement is NOT true about the Diels-Alder reaction?
A) It is a [4+2] cycloaddition reaction.
B) The diene must be in the s-cis conformation to react.
C) Most Diels-Alder reactions are reversible.
D) It is a sigmatropic rearrangement.
E) Electron donating groups on the diene and electron withdrawing groups on the dienophile favor product formation.
A) It is a [4+2] cycloaddition reaction.
B) The diene must be in the s-cis conformation to react.
C) Most Diels-Alder reactions are reversible.
D) It is a sigmatropic rearrangement.
E) Electron donating groups on the diene and electron withdrawing groups on the dienophile favor product formation.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the pericyclic reaction in which two sigma bonds are formed and two pi bonds are broken.
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the structure for 1,2 addition product for the following reaction and explain why it is a major product rather than 1,4 addition product. 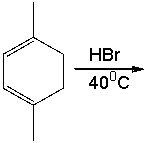


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following dienophiles is most reactive in the Diels-Alder reaction? 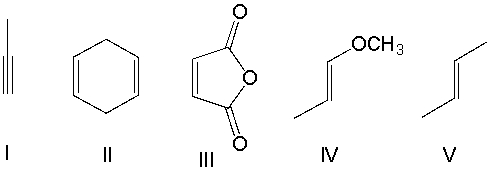
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
The addition of a _____________ bond improves the elasticity in synthetic rubber.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
What is the correct classification of the following pericyclic reaction? 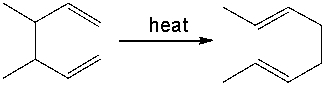
A) electrophilic addition
B) sigmatropic rearrangement
C) cycloaddition
D) electrocyclic reaction
E) C and D
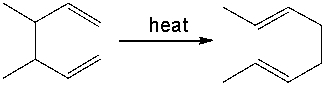
A) electrophilic addition
B) sigmatropic rearrangement
C) cycloaddition
D) electrocyclic reaction
E) C and D

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
The Diels Alder reaction is a concerted reaction. Define concerted.
A) The product contains a cyclic ring.
B) The diene must be in the s-cis conformation to react.
C) All changes in bonding (bond making and bond breaking) occur simultaneously.
D) It is an endothermic reaction.
E) Both exo and endo products are formed.
A) The product contains a cyclic ring.
B) The diene must be in the s-cis conformation to react.
C) All changes in bonding (bond making and bond breaking) occur simultaneously.
D) It is an endothermic reaction.
E) Both exo and endo products are formed.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
What is the correct classification of the following pericyclic reaction? 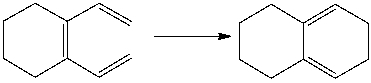
A) electrophilic addition
B) sigmatropic rearrangement
C) cycloaddition
D) electrocyclic reaction
E) C & D

A) electrophilic addition
B) sigmatropic rearrangement
C) cycloaddition
D) electrocyclic reaction
E) C & D

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is true of pericyclic reactions?
A) they are concerted reactions
B) proceed via a cyclic transition state
C) no intermediates are formed
D) all of these
A) they are concerted reactions
B) proceed via a cyclic transition state
C) no intermediates are formed
D) all of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


