Deck 23: Introduction to Organometallic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 23: Introduction to Organometallic Compounds
1
Which of the following compounds has the most nucleophilic carbon atom?
A) CH3Cl
B) CH3Li
C) (CH3)2Zn
D) (CH3)2Cu
A) CH3Cl
B) CH3Li
C) (CH3)2Zn
D) (CH3)2Cu
CH3Li
2
Explain why the solvent for a Grignard reaction must be free of water.
Water consumes the Grignard reagent.
3
Which of the following solvents is/are good solvents to be used in the preparation of organometallic reagents such as organolithiums and Grignard reagents? Select all that apply.
A) ethanol
B) diethyl ether
C) cyclohexanol
D) tetrahydrofuran
E) dioxane
A) ethanol
B) diethyl ether
C) cyclohexanol
D) tetrahydrofuran
E) dioxane
diethyl ether
tetrahydrofuran
dioxane
tetrahydrofuran
dioxane
4
Identify the product when 2-pentanone reacts with one equivalent of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the product when butanal reacts with one equivalent of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
What functional group most typically results when a Grignard Reagent reacts with an acid halide, after aqueous workup?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following functional groups does not afford an alcohol when treated with a Grignard reagent followed by a protic work-up.
A) ketone
B) aldehyde
C) ester
D) epoxide
E) nitrile
A) ketone
B) aldehyde
C) ester
D) epoxide
E) nitrile

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Which alkyl halide is most reactive with magnesium metal?
A) CH3CH2CH2Br
B) CH3CH2CH2F
C) CH3CH2CH2I
D) CH3CH2CH2Cl
A) CH3CH2CH2Br
B) CH3CH2CH2F
C) CH3CH2CH2I
D) CH3CH2CH2Cl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Diethyl ether is a good solvent to be used with Grignard reagents. Which of the following explanations for this fact is false?
A) It has a very high boiling so it can be heated to drive the Grignard formation to completion.
B) It can serve as a Lewis base and stabilize the Grignard reagent.
C) It is polar enough to stabilize the Grignard reagent.
D) It is aprotic and thus is not acidic enough to react with the Grignard reagent.
A) It has a very high boiling so it can be heated to drive the Grignard formation to completion.
B) It can serve as a Lewis base and stabilize the Grignard reagent.
C) It is polar enough to stabilize the Grignard reagent.
D) It is aprotic and thus is not acidic enough to react with the Grignard reagent.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
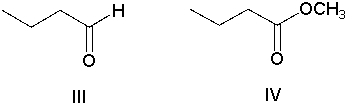
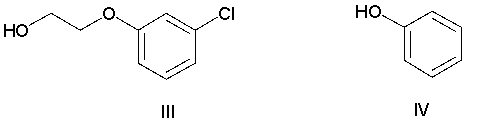
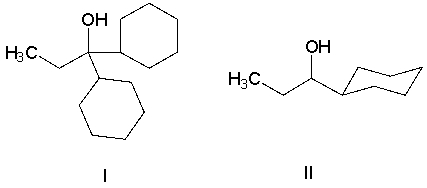
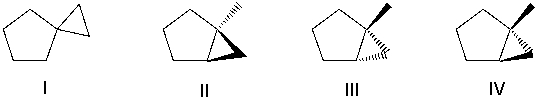
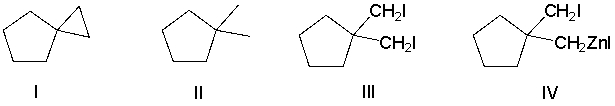
Which of the following compounds has the most nucleophilic carbon atom? 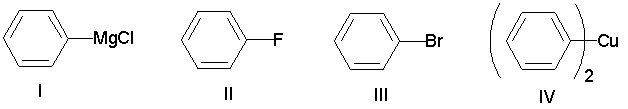
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the product when formaldehyde reacts with one equivalent of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds has the most ionic character in the C-M bond?
A) HC≡CMgBr
B) HC≡CK
C) HC≡CNa
D) HC≡CLi
A) HC≡CMgBr
B) HC≡CK
C) HC≡CNa
D) HC≡CLi

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Which alkyl halide is least reactive with magnesium metal?
A) CH3CH2CH2Br
B) CH3CH2CH2F
C) CH3CH2CH2I
D) CH3CH2CH2Cl
A) CH3CH2CH2Br
B) CH3CH2CH2F
C) CH3CH2CH2I
D) CH3CH2CH2Cl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the product when carbon dioxide reacts with one equivalent of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
What functional group typically results when an organocopper compound (lithium organocuprate) reacts with an acid chloride?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds has the least nucleophilic carbon atom?
A) CH3CH2MgBr
B) CH3CH2MgI
C) (CH3CH2)2Zn
D) (CH3CH2)2Cu
A) CH3CH2MgBr
B) CH3CH2MgI
C) (CH3CH2)2Zn
D) (CH3CH2)2Cu

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is false regarding the preparation of organolithium compounds?
A) Organolithium compounds can be made from alkyl halides (R-X).
B) Organolithium compounds can be made from aryl halides (Ar-X).
C) Vinyl halides cannot be used to form vinyl lithium reagents.
D) Lithium halides are byproducts of the reaction of lithium metal with alkyl halides.
E) It is necessary to use two equivalents of lithium metal.
A) Organolithium compounds can be made from alkyl halides (R-X).
B) Organolithium compounds can be made from aryl halides (Ar-X).
C) Vinyl halides cannot be used to form vinyl lithium reagents.
D) Lithium halides are byproducts of the reaction of lithium metal with alkyl halides.
E) It is necessary to use two equivalents of lithium metal.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the most polar bond?
A) C-B
B) C-Pd
C) C-Zn
D) C-Pt
A) C-B
B) C-Pd
C) C-Zn
D) C-Pt

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the product when ethylene oxide reacts with one equivalent of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
Why is ethanol not a viable solvent in the synthesis of Grignard reagents?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
What is the name of the product of the following reaction sequence? 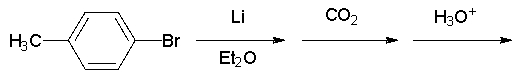
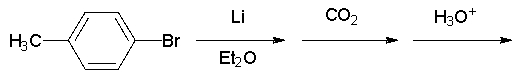

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
Give the final product. 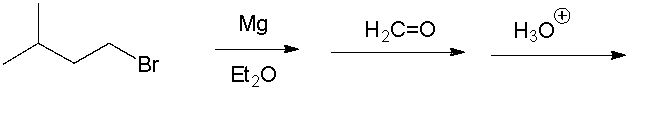


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
What is the product of the following sequence? 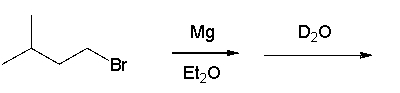


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Give the final product. 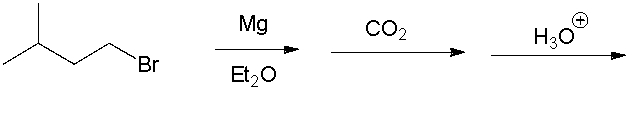


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Which reactant would afford the product shown as the major isomeric product? 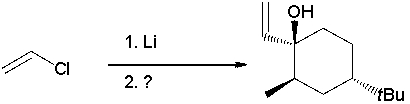
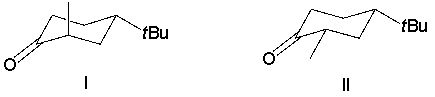
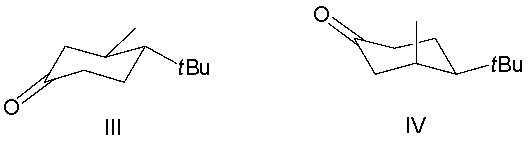
A) I
B) II
C) III
D) IV
E) none of the above

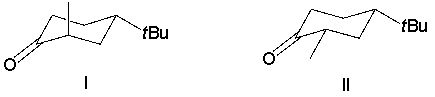

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following reagents cannot be used in the synthesis of a Gilman reagent?
A) lithium metal
B) lithium halide
C) alkyl halide
D) aryl halide
E) vinyl halide
A) lithium metal
B) lithium halide
C) alkyl halide
D) aryl halide
E) vinyl halide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Identify the starting material. 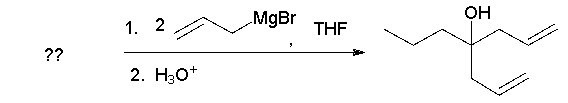
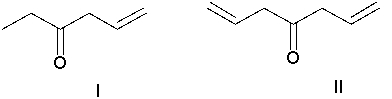
A) I
B) II
C) III
D) IV
E) The product cannot be formed directly from one of the reactants above.



A) I
B) II
C) III
D) IV
E) The product cannot be formed directly from one of the reactants above.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
Give the final product. 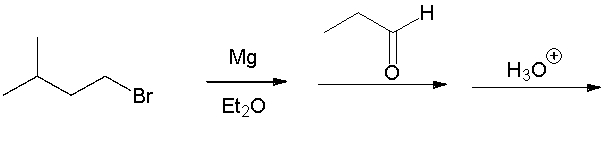


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following electrophiles are commonly used with Gilman reagents to form C-C bonds? Select all that apply.
A)acid chlorides
B)α,β-unsaturated ketones
C)esters
D)alkyl halides
E)amides
A)acid chlorides
B)α,β-unsaturated ketones
C)esters
D)alkyl halides
E)amides

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the following sequence? 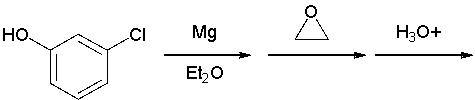
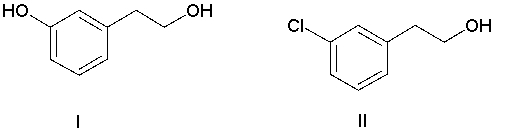
A)I
B)II
C)III
D)IV
E)none of the them
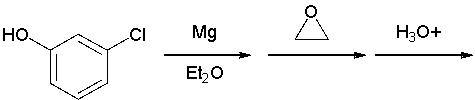


A)I
B)II
C)III
D)IV
E)none of the them

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the product when an ester reacts with two equivalents of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the product when a nitrile reacts with two equivalent of a Grignard reagents.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
What is the product of the following sequence? 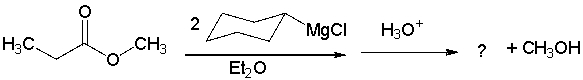
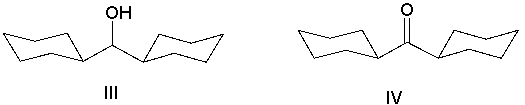
A) I
B) II
C) III
D) IV



A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
Starting from bromobenzene and any other reagents with two carbons or fewer, propose a synthesis of the compound shown. 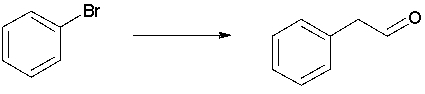


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
What is the product of the following sequence? 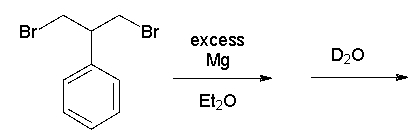
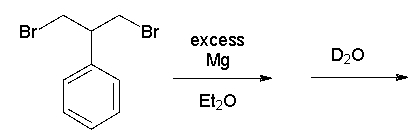

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements is(are) true regarding the preparation of Gilman reagents?
A) Gilman reagents can be made from alkyl halides (R-X).
B) Gilman reagents can be made from aryl halides (Ar-X).
C) Gilman reagents can be made from organolithium reagents.
D) Lithium halides are byproducts of the reaction to make Gilman reagents.
E) all of the above
A) Gilman reagents can be made from alkyl halides (R-X).
B) Gilman reagents can be made from aryl halides (Ar-X).
C) Gilman reagents can be made from organolithium reagents.
D) Lithium halides are byproducts of the reaction to make Gilman reagents.
E) all of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
Give the final product. 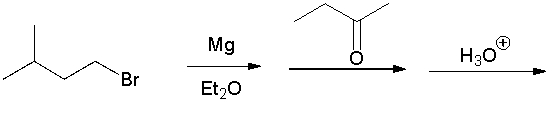
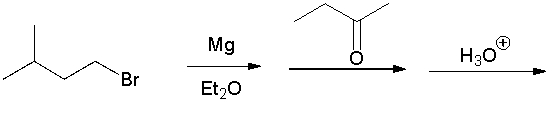

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
What is the product of the following sequence? 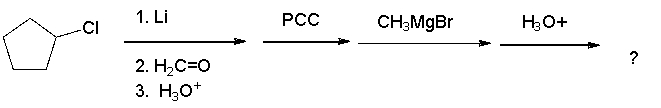
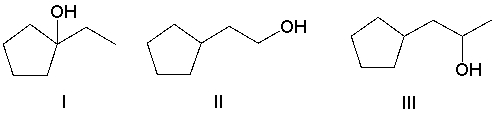
A) I
B) II
C) III
D) IV
E) V



A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following electrophiles are commonly used with Gilman reagents to form C-C bonds?
A) alkyl halides
B) aryl halides
C) vinyl halides
D) acyl halides
E) all the above
A) alkyl halides
B) aryl halides
C) vinyl halides
D) acyl halides
E) all the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the product when a nitrile reacts with one equivalent of a Grignard reagent.
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) carboxylic acid
E) ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
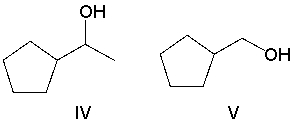
What product(s) result in the following reaction? Select all that apply. 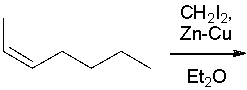
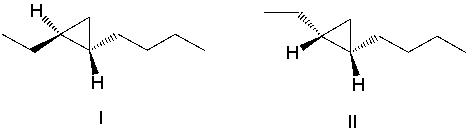
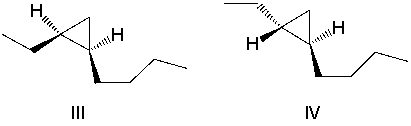
A)I
B) II
C)III
D)IV



A)I
B) II
C)III
D)IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
A total synthesis of ingenol utilized the Corey-Posner/Whitesides-House coupling reaction in a key C-C bond forming step (J. Am. Chem. Soc. 2002, 124, 9726). Propose a reagent to perform this transformation. 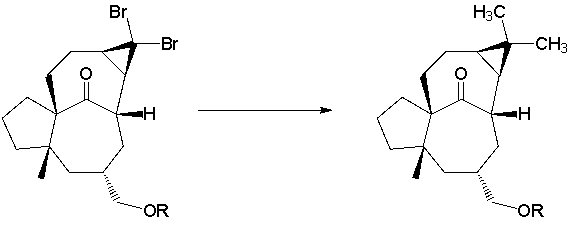


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
What is the product of the following reaction? 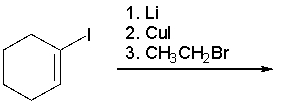


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Gillman reagents are intolerant to the presence of what functional group?
A) ketone
B) ester
C) amide
D) All of the functional groups above are compatible with Gilman reagents.
A) ketone
B) ester
C) amide
D) All of the functional groups above are compatible with Gilman reagents.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Starting from vinyl chloride and ________ as the only source of carbon atoms, 1-heptene can be synthesized using a Corey-Posner/Whitesides-House coupling reaction.
A) 1-bromopropane
B) 2-bromopentane
C) 1-bromohexane
D) 1-bromopentane
A) 1-bromopropane
B) 2-bromopentane
C) 1-bromohexane
D) 1-bromopentane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following Gilman reagents could be used with a vinyl halide to afford the (E,E)-diene shown below? Select all that apply. 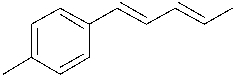
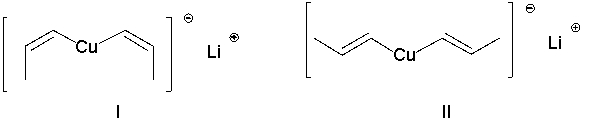
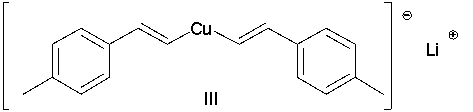
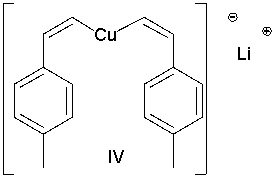
A) I
B) II
C) III
D) IV
E) II and III




A) I
B) II
C) III
D) IV
E) II and III

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
What is the product of the following reaction? 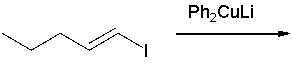
A) (E)-1-phenyl-1-pentene
B) (Z)-1-phenyl-1-pentene
C) (E)-1-phenyl-2-pentene
D) (Z)-1-phenyl-2-pentene
E) 2-phenyl-1-pentene

A) (E)-1-phenyl-1-pentene
B) (Z)-1-phenyl-1-pentene
C) (E)-1-phenyl-2-pentene
D) (Z)-1-phenyl-2-pentene
E) 2-phenyl-1-pentene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements is false regarding the reactivity of Gilman reagents?
A) They react with acyl halides to give ketones.
B) They react with α,β-unsaturated ketones to give alcohols.
C) They react with alkyl halides or aryl halides in a coupling reaction.
D) They are unreactive with tertiary halides.
A) They react with acyl halides to give ketones.
B) They react with α,β-unsaturated ketones to give alcohols.
C) They react with alkyl halides or aryl halides in a coupling reaction.
D) They are unreactive with tertiary halides.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following statements is false?
A) Carbenes are generally long lived.
B) Carbenes have a carbon with non-bonding electrons.
C) Carbenes have a carbon missing an octet.
D) Carbenes can be formed by α-elimination.
E) Carbenes are electrophilic.
A) Carbenes are generally long lived.
B) Carbenes have a carbon with non-bonding electrons.
C) Carbenes have a carbon missing an octet.
D) Carbenes can be formed by α-elimination.
E) Carbenes are electrophilic.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
What product(s) result in the following reaction? Select all that apply. 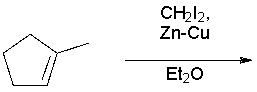
A)I
B)II
C)III
D)IV
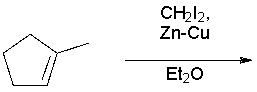

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following would react fastest in the Corey-Posner/Whitesides-House reaction?
A) cyclohexyl chloride
B) cyclohexyl iodide
C) tert-butyl chloride
D) tert-butyl iodide
A) cyclohexyl chloride
B) cyclohexyl iodide
C) tert-butyl chloride
D) tert-butyl iodide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
What are the conditions for the following reaction? 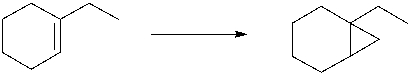


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is a Simmons-Smith cyclopropanation?
A) R-X + Zn →
B) R2C=CR2 + CH2I2 + Zn-Cu →
C) CHCl3 + NaOH →
D) R2C=CHI + (CH3)2CuLi →
A) R-X + Zn →
B) R2C=CR2 + CH2I2 + Zn-Cu →
C) CHCl3 + NaOH →
D) R2C=CHI + (CH3)2CuLi →

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
The following synthesis is not likely to work. Which of the following explains why? 
A) Lithium cannot react with aryl halides to form organolithium compounds.
B) CH3CH2Br does not do cross coupling in Corey-Posner/Whitesides-House reactions.
C) The cuprate formed would react with the ketone.
D) The organolithium formed would react with the ketone.

A) Lithium cannot react with aryl halides to form organolithium compounds.
B) CH3CH2Br does not do cross coupling in Corey-Posner/Whitesides-House reactions.
C) The cuprate formed would react with the ketone.
D) The organolithium formed would react with the ketone.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Identify the best reagent for the reaction below. 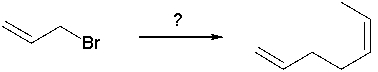
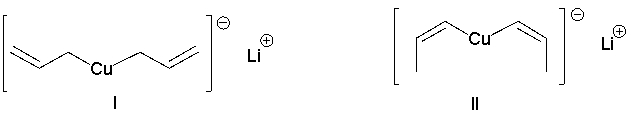

A) I
B) II
C) III
D) IV
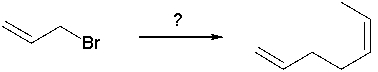
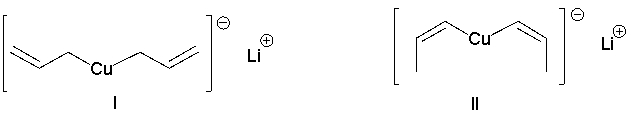

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
Starting from bromobenzene and ________ as the only source of carbon atoms, 1-phenylcyclohexene can be synthesized using a Corey-Posner/Whitesides-House coupling reaction.
A) 1-iodocyclohexene
B) 3-iodocyclohexene
C) iodocyclohexane
D) (iodomethyl)cyclohexane
A) 1-iodocyclohexene
B) 3-iodocyclohexene
C) iodocyclohexane
D) (iodomethyl)cyclohexane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
The Corey-Posner/Whitesides-House reaction couples a lithium dialkyl cuprate with all but which of the following?
A) primary alkyl halides
B) tertiary alkyl halides
C) aryl halides
D) methyl halides
E) vinyl halides
A) primary alkyl halides
B) tertiary alkyl halides
C) aryl halides
D) methyl halides
E) vinyl halides

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
What product(s) result in the following reaction? Select all that apply. 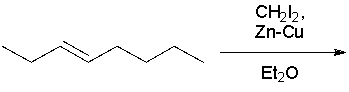
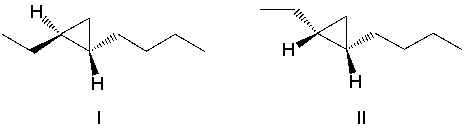
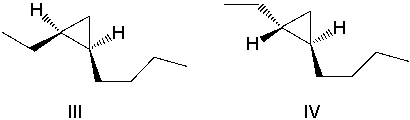
A)I
B)II
C)III
D)IV



A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
What product results in the following reaction? 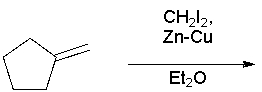
A) I
B) II
C) III
D) IV
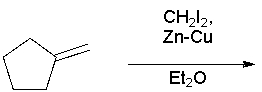

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
The Simmons-Smith reaction involves the formation of _____?
A) alkenes
B) alkynes
C) cyclopropanes
D) cuprates
A) alkenes
B) alkynes
C) cyclopropanes
D) cuprates

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Elaboration of an advanced intermediate toward the synthesis of the pulmoside aglycon is shown below. Give the structures of the intermediates A-C. (J. Am. Chem. Soc. 2013, 135, 2501.) 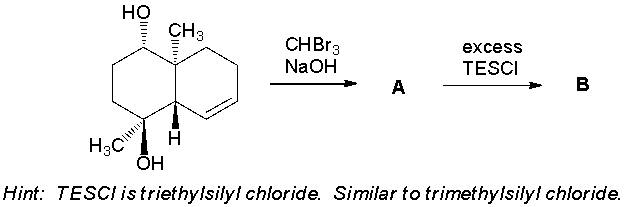
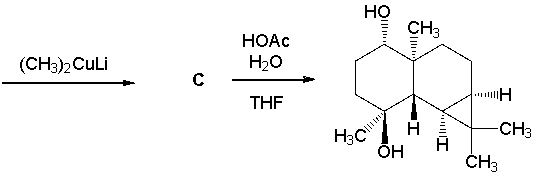



Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
The reaction of a carbene with an alkene to make a cyclopropane commonly goes through which type mechanism?
A) proton transfer
B) loss of leaving group
C) cycloaddition
D) α-elimination
A) proton transfer
B) loss of leaving group
C) cycloaddition
D) α-elimination

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
What is the product of the following reaction? 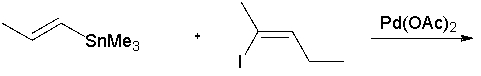
A) (2E,4Z)-4-methylhepta-2,4-diene
B) (2Z,4E)-4-methylhepta-2,4-diene
C) (3E,5Z)-4-methylhepta-3,5-diene
D) (3Z,5Z)-4-methylhepta-3,5-diene
E) (3E,5E)-4-methylhepta-3,5-diene

A) (2E,4Z)-4-methylhepta-2,4-diene
B) (2Z,4E)-4-methylhepta-2,4-diene
C) (3E,5Z)-4-methylhepta-3,5-diene
D) (3Z,5Z)-4-methylhepta-3,5-diene
E) (3E,5E)-4-methylhepta-3,5-diene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
The Stille reaction involves coupling of which pair of reagents?
A) organohalide and organotin
B) organohalide and organoboron
C) organotin and organozinc
D) organoboron and organozinc
A) organohalide and organotin
B) organohalide and organoboron
C) organotin and organozinc
D) organoboron and organozinc

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
A report on the synthesis of Aromatic Erythrina Alkaloids uses the following transformation (Org. Lett. 2006, 8, 2143). Propose reasonable reaction conditions. 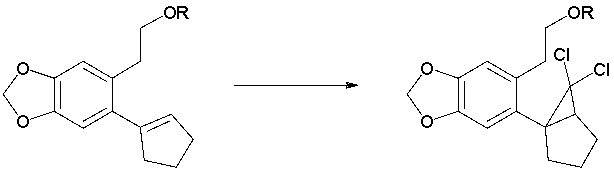


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following R groups (R-SnBu3) would undergo the fastest transmetallation in a Stille coupling reaction?
A) alkyl
B) aryl
C) vinyl
D) benzyl
A) alkyl
B) aryl
C) vinyl
D) benzyl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following R groups (R-SnBu3) would undergo the slowest transmetallation in a Stille coupling reaction?
A) alkyl
B) aryl
C) vinyl
D) benzyl
A) alkyl
B) aryl
C) vinyl
D) benzyl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Which catalysts are common in the Stille coupling reaction?
A) Pd(PPh3)4
B) Pd (OAc)2
C) PdCl2(PPh3)2
D) All of the above
A) Pd(PPh3)4
B) Pd (OAc)2
C) PdCl2(PPh3)2
D) All of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
The Stille reaction couples organotin reagents with all but one of the following. Which is not a common coupling partner?
A) -I
B) -Br
C) -Cl
D) -F
E) -OTf
A) -I
B) -Br
C) -Cl
D) -F
E) -OTf

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the product(s) of the following reaction: 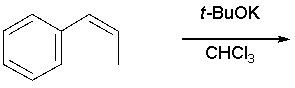
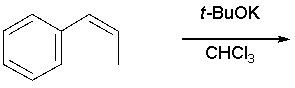

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
What is the product in the following reaction? 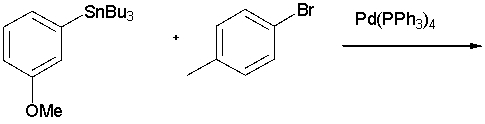
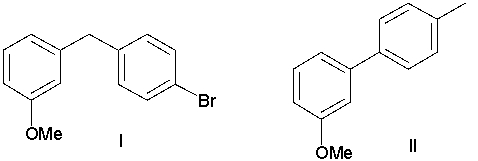
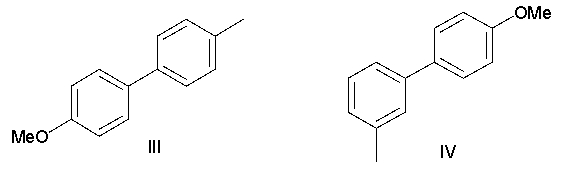
A) I
B) II
C) III
D) IV



A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
In a total synthesis of the bisnortriterpenoid rubriflordilactone A, a key step involved the following transformation. Draw the product. (J. Am. Chem. Soc. 2014, 136, 16477). 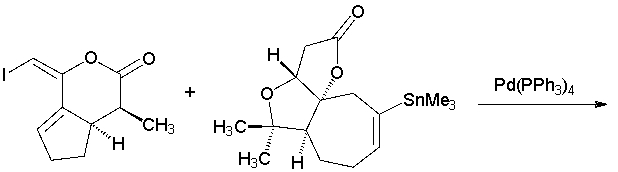


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is a boronic acid? 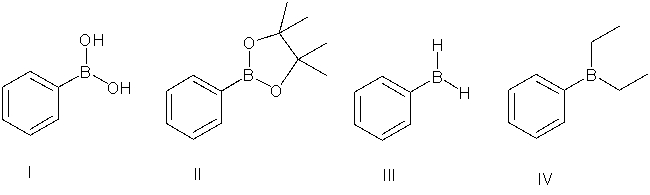
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the product(s) of the following reaction: 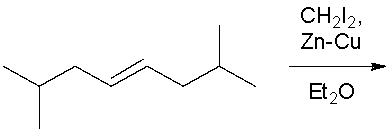


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Treatment of chloroform (CHCl3) with strong bases results in _______ which is mechanistically described as a_______.
A) α-elimination; loss of leaving group followed by deprotonation.
B) α-elimination; deprotonation followed by a loss of leaving group.
C) β-elimination; deprotonation followed by loss of leaving group.
D) β-elimination; concerted deprotonation and loss of leaving group.
A) α-elimination; loss of leaving group followed by deprotonation.
B) α-elimination; deprotonation followed by a loss of leaving group.
C) β-elimination; deprotonation followed by loss of leaving group.
D) β-elimination; concerted deprotonation and loss of leaving group.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
What is the product of the following reaction? 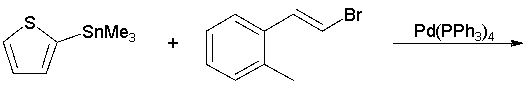
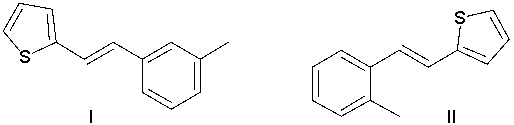
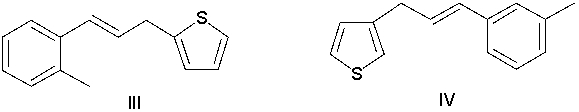
A) I
B) II
C) III
D) IV



A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is not a mechanistic step in the generally accepted mechanism of the Stille coupling?
A) transmetallation
B) reductive elimination
C) syn-elimination
D) oxidative addition
A) transmetallation
B) reductive elimination
C) syn-elimination
D) oxidative addition

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is a boronic ester? 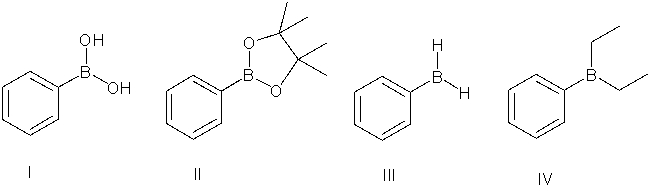
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Biselyngbyolide A is a cytotoxic natural product that shows promise for the treatment of bone lytic diseases. Shown below is a recent synthesis of this molecule (Org. Lett. 2014, 16, 2858). Give the structure of intermediate 1 and Biselyngbyolide
A. Hint: PPTS is a strong organic acid.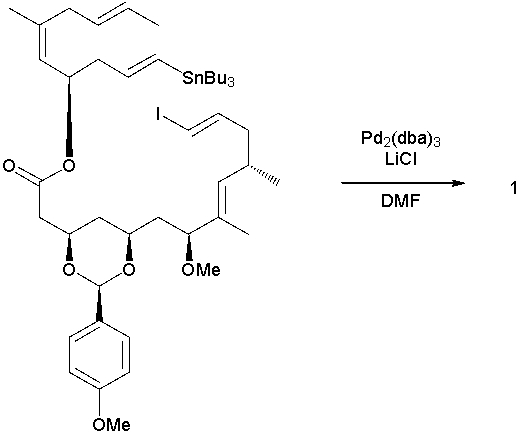
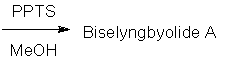
A. Hint: PPTS is a strong organic acid.

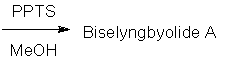

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
A total synthesis of ingenol utilized a dibromocyclopropanation via a carbene intermediate (J. Am. Chem. Soc. 2002, 124, 9726). What key type of reagent is missing from the reaction conditions? 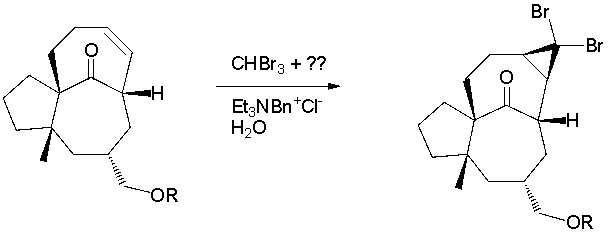
A) Bronsted Acid
B) Bronsted Base
C) radical initiator
D) Lewis Acid catalyst

A) Bronsted Acid
B) Bronsted Base
C) radical initiator
D) Lewis Acid catalyst

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


