Deck 5: Protein Purification and Characterization Techniques
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 5: Protein Purification and Characterization Techniques
1
Which would be best to separate positively charged proteins?
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
C
2
The purity of an enzyme at various stages of purification is best measured by
A) total protein.
B) total enzyme activity.
C) specific activity of the enzyme.
D) percent recovery of the protein.
E) percent recovery of the enzyme.
A) total protein.
B) total enzyme activity.
C) specific activity of the enzyme.
D) percent recovery of the protein.
E) percent recovery of the enzyme.
C
3
In gel filtration chromatography
A) materials are separated based on their size, the smaller ones eluting first.
B) materials are separated based on their size, the larger ones eluting first.
C) materials are separated based on their hydrophobic nature, the more hydrophobic ones eluting first.
D) materials are separated based on their hydrophobic nature, the less hydrophobic ones eluting first.
A) materials are separated based on their size, the smaller ones eluting first.
B) materials are separated based on their size, the larger ones eluting first.
C) materials are separated based on their hydrophobic nature, the more hydrophobic ones eluting first.
D) materials are separated based on their hydrophobic nature, the less hydrophobic ones eluting first.
B
4
Salting out with ammonium sulfate is based upon proteins interacting with other proteins via
A) hydrogen bonds.
B) ionic bonds.
C) hydrophobic interactions.
D) disulfide bonds.
A) hydrogen bonds.
B) ionic bonds.
C) hydrophobic interactions.
D) disulfide bonds.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Using differential centrifugation it is possible to separate
A) nuclei, mitochondria, and ribosomes into three separate fractions
B) organelles from contaminating salts
C) proteins that differ in charge
D) proteins from membranes
A) nuclei, mitochondria, and ribosomes into three separate fractions
B) organelles from contaminating salts
C) proteins that differ in charge
D) proteins from membranes

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Which type of column is most affected by the shape of the protein,for example,comparing spherical and cigar-shaped proteins?
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following happens as a protein is purified?
A) the percent recovery and the fold purification both increase
B) the percent recovery and the fold purification both decrease
C) the percent recovery increases and the fold purification decreases
D) the percent recovery decreases and the fold purification increases
A) the percent recovery and the fold purification both increase
B) the percent recovery and the fold purification both decrease
C) the percent recovery increases and the fold purification decreases
D) the percent recovery decreases and the fold purification increases

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
What tends to happen to the percent recovery during a protein's purification?
A) The number usually steadily increases during the purification.
B) The number usually steadily decreases during the purification.
C) The number usually stays fairly constant during the purification.
D) There is no general trend for percent recovery during a protein purification.
A) The number usually steadily increases during the purification.
B) The number usually steadily decreases during the purification.
C) The number usually stays fairly constant during the purification.
D) There is no general trend for percent recovery during a protein purification.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
The typical order for the major steps of enzyme isolation would be (from first to last):
A) Homogenization, salt fractionation, electrophoresis, column chromatography.
B) Homogenization, column chromatography, salt fractionation, electrophoresis.
C) Homogenization, salt fractionation, column chromatography, electrophoresis.
D) Salt fractionation, homogenization, electrophoresis, column chromatography.
E) Homogenization, electrophoresis, salt fractionation, column chromatography.
A) Homogenization, salt fractionation, electrophoresis, column chromatography.
B) Homogenization, column chromatography, salt fractionation, electrophoresis.
C) Homogenization, salt fractionation, column chromatography, electrophoresis.
D) Salt fractionation, homogenization, electrophoresis, column chromatography.
E) Homogenization, electrophoresis, salt fractionation, column chromatography.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
Elution of proteins by means of a pH gradient would work best with this type of column:
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
Ammonium sulfate is useful in protein purification because
A) it contains nitrogen and sulfur, both of which occur in proteins
B) it is sparingly soluble in water, causing proteins to co-precipitate with it
C) very pure proteins are obtained when it is used
D) it forms ion-dipole interactions with water, making proteins less soluble and more likely to precipitate
A) it contains nitrogen and sulfur, both of which occur in proteins
B) it is sparingly soluble in water, causing proteins to co-precipitate with it
C) very pure proteins are obtained when it is used
D) it forms ion-dipole interactions with water, making proteins less soluble and more likely to precipitate

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
Which would be best to separate a protein that binds strongly to its substrate?
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
Which separates based on the ionic charge on a protein?
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
Which would be best to separate proteins of similar size?
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
Differential centrifugation at low speeds (several thousand RPM) is a useful step when
A) organelles have been lysed.
B) enzymes of interest have different sizes.
C) cell membranes must be left intact.
D) ribosomes need to be broken down.
E) there are either organelles or debris to separate.
A) organelles have been lysed.
B) enzymes of interest have different sizes.
C) cell membranes must be left intact.
D) ribosomes need to be broken down.
E) there are either organelles or debris to separate.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
In affinity chromatography,a protein
A) which binds to the ligand will remain on the column.
B) which binds to the ligand will elute from the column.
C) which is hydrophobic will remain on the column.
D) which is hydrophilic will remain on the column.
A) which binds to the ligand will remain on the column.
B) which binds to the ligand will elute from the column.
C) which is hydrophobic will remain on the column.
D) which is hydrophilic will remain on the column.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following are principles on which to base column chromatography?
A) Molecular size
B) Isoionic pH or pI
C) Ion exchange
D) Both molecular size and ion exchange
E) All of these
A) Molecular size
B) Isoionic pH or pI
C) Ion exchange
D) Both molecular size and ion exchange
E) All of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
The following methods are useful for cell homogenization:
A) Sonication.
B) Freezing and thawing.
C) Detergents.
D) Enzymes.
E) All of these are correct.
A) Sonication.
B) Freezing and thawing.
C) Detergents.
D) Enzymes.
E) All of these are correct.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
The typical order of differential centrifugation for organelles is (from slowest speed/lowest g to fastest speed/highest g):
A) nuclei, microsomes, mitochondria & chloroplasts, cytosol, whole cells
B) whole cells, nuclei, mitochondria & chloroplasts, microsomes, cytosol
C) cytosol, microsomes, nuclei, mitochondria & chloroplasts, whole cells
D) nuclei, mitochondria & chloroplasts, whole cells, cytosol, microsomes
E) whole cells, cytosol, microsomes, nuclei, mitochondria & chloroplasts
A) nuclei, microsomes, mitochondria & chloroplasts, cytosol, whole cells
B) whole cells, nuclei, mitochondria & chloroplasts, microsomes, cytosol
C) cytosol, microsomes, nuclei, mitochondria & chloroplasts, whole cells
D) nuclei, mitochondria & chloroplasts, whole cells, cytosol, microsomes
E) whole cells, cytosol, microsomes, nuclei, mitochondria & chloroplasts

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
Which separates on the basis of molecular weight?
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange
A) Gel filtration
B) Affinity chromatography
C) Cation exchange
D) Anion exchange
E) Cation or anion exchange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
The degree of separation in molecular sieve chromatography depends on
A) the polarity of the mobile phase
B) the pKa of the buffer material in the mobile phase
C) the chemical nature of the sieve material
D) the size of the pores in the sieve material
A) the polarity of the mobile phase
B) the pKa of the buffer material in the mobile phase
C) the chemical nature of the sieve material
D) the size of the pores in the sieve material

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
In a sample consisting of lysine,leucine,and glutamic acid,which will be eluted last from an anion exchange resin at pH 7?
A) all three will be eluted at the same time
B) lysine
C) leucine
D) glutamic acid
A) all three will be eluted at the same time
B) lysine
C) leucine
D) glutamic acid

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
In the SDS-PAGE (sodium dodecylsulfate - polyacrylamide gel electrophoresis) method,separation takes place on the basis of
A) charge only, because all particles have different charges, but the same mass.
B) the sieving action of the gel, because all particles have the same charge, but different masses.
C) the sieving action of the gel, because all particles have approximately the same charge/mass ratio, but different masses.
D) the chemical nature of the buffer used as the electrolyte.
A) charge only, because all particles have different charges, but the same mass.
B) the sieving action of the gel, because all particles have the same charge, but different masses.
C) the sieving action of the gel, because all particles have approximately the same charge/mass ratio, but different masses.
D) the chemical nature of the buffer used as the electrolyte.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 5A The following diagram shows the anode,cathode,and pH gradient of an isoelectric focusing bed:
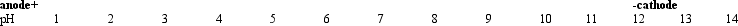 Refer to Exhibit 5A.A mixture of asp,asn,and arg is placed in the bed where the pH is 7,and the current is turned on.From left to right,which best represents the final positions of the individual amino acids?
Refer to Exhibit 5A.A mixture of asp,asn,and arg is placed in the bed where the pH is 7,and the current is turned on.From left to right,which best represents the final positions of the individual amino acids?
A) asp asn arg
B) arg asn asp
C) asn asp arg
D) arg asp asn
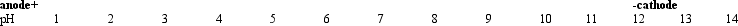 Refer to Exhibit 5A.A mixture of asp,asn,and arg is placed in the bed where the pH is 7,and the current is turned on.From left to right,which best represents the final positions of the individual amino acids?
Refer to Exhibit 5A.A mixture of asp,asn,and arg is placed in the bed where the pH is 7,and the current is turned on.From left to right,which best represents the final positions of the individual amino acids?A) asp asn arg
B) arg asn asp
C) asn asp arg
D) arg asp asn

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
The order of elution of AAs H,E,& K from a cation exchange column by a pH 6 buffer is
A) H E K
B) E H K
C) K H E
D) E K H
A) H E K
B) E H K
C) K H E
D) E K H

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
In electrophoresis experiments
A) the separation must be carried out in bright light
B) the polarity of substances to be separated is more important than their charge or size
C) the sample can be badly degraded as a result of the separation
D) an electric field must be applied to the mixture to be separated
A) the separation must be carried out in bright light
B) the polarity of substances to be separated is more important than their charge or size
C) the sample can be badly degraded as a result of the separation
D) an electric field must be applied to the mixture to be separated

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 5A The following diagram shows the anode,cathode,and pH gradient of an isoelectric focusing bed:
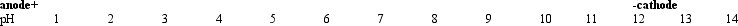 Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 11,and the current were turned on,it would migrate closest to which of the following positions?
Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 11,and the current were turned on,it would migrate closest to which of the following positions?
A) pH 4
B) pH 6
C) pH 8
D) pH 10
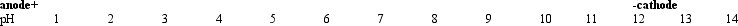 Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 11,and the current were turned on,it would migrate closest to which of the following positions?
Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 11,and the current were turned on,it would migrate closest to which of the following positions?A) pH 4
B) pH 6
C) pH 8
D) pH 10

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
How many bands would be produced when hemoglobin is subjected to SDS-PAGE?
A) 1
B) 2
C) 3
D) 4
E) 2, but only if the size of the pores in the gel would allow two proteins of slightly different size to be separated
A) 1
B) 2
C) 3
D) 4
E) 2, but only if the size of the pores in the gel would allow two proteins of slightly different size to be separated

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
The following are all principles on which to base electrophoresis except:
A) Molecular size
B) Isoionic pH or pI
C) Net charge
D) Binding to a substrate
E) Shape
A) Molecular size
B) Isoionic pH or pI
C) Net charge
D) Binding to a substrate
E) Shape

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
In all forms of chromatography one way of identifying eluted substances is by
A) fluorescence spectroscopy
B) comparison with standards
C) radioactive labeling
D) treating fractions with a reagent that will cause a color change
A) fluorescence spectroscopy
B) comparison with standards
C) radioactive labeling
D) treating fractions with a reagent that will cause a color change

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
Which substance would you expect to be eluted first from a molecular sieve column with a suitable degree of crosslinking?
A) hemoglobin
B) myoglobin
C) 2,3-bisphosphoglycerate
D) all would elute at the same rate
A) hemoglobin
B) myoglobin
C) 2,3-bisphosphoglycerate
D) all would elute at the same rate

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not an example of column chromatography?
A) ammonium sulfate fractionation
B) ion-exchange separation
C) HPLC
D) affinity separation
A) ammonium sulfate fractionation
B) ion-exchange separation
C) HPLC
D) affinity separation

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
In any form of chromatography,how will a compound which interacts more strongly with the stationary phase elute compared to one that interacts less strongly?
A) A compound interacting more strongly will elute earlier than one with weaker interactions.
B) A compound interacting more strongly will elute later than one with weaker interactions.
C) The order of elution has nothing to do with interactions with the stationary phase, but with interactions with the mobile phase.
A) A compound interacting more strongly will elute earlier than one with weaker interactions.
B) A compound interacting more strongly will elute later than one with weaker interactions.
C) The order of elution has nothing to do with interactions with the stationary phase, but with interactions with the mobile phase.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Exhibit 5A The following diagram shows the anode,cathode,and pH gradient of an isoelectric focusing bed:
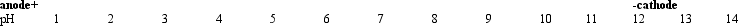 Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 7,and the current were turned on,it would migrate closest to which of the following positions?
Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 7,and the current were turned on,it would migrate closest to which of the following positions?
A) pH 4
B) pH 6
C) pH 8
D) pH 10
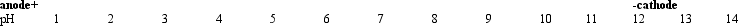 Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 7,and the current were turned on,it would migrate closest to which of the following positions?
Refer to Exhibit 5A.If the amino acid glycine were placed in the bed where the pH is 7,and the current were turned on,it would migrate closest to which of the following positions?A) pH 4
B) pH 6
C) pH 8
D) pH 10

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
In chromatography the experimental setup always requires
A) a stationary phase and a mobile phase
B) a spectrophotometric detecting device
C) a sample in which components differ in charge
D) a sample in which components differ in polarity
A) a stationary phase and a mobile phase
B) a spectrophotometric detecting device
C) a sample in which components differ in charge
D) a sample in which components differ in polarity

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
In affinity chromatography
A) there is nonspecific binding of proteins to column material
B) only minor purifications can be obtained
C) the mobile phase is always pure water
D) the ligand is alwayse specific for one type of protein to be bound
E) there can be molecule specific ligands or group specific ligands
A) there is nonspecific binding of proteins to column material
B) only minor purifications can be obtained
C) the mobile phase is always pure water
D) the ligand is alwayse specific for one type of protein to be bound
E) there can be molecule specific ligands or group specific ligands

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
A chromatography technique where a solution of nonpolar compounds is put through a column that has a nonpolar liquid immobilized on an inert matrix is which type of chromatography?
A) Gel filtration
B) Ion exchange
C) Affinity
D) HPLC
E) Reverse Phase HPLC
A) Gel filtration
B) Ion exchange
C) Affinity
D) HPLC
E) Reverse Phase HPLC

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Two-dimensional electrophoresis usually exploits these 2 different properties of proteins:
A) Molecular weight and shape
B) Molecular weight and net charge
C) Molecular weight and isoionic pH
D) Isoionic pH and shape
E) Isoionic pH and net charge
A) Molecular weight and shape
B) Molecular weight and net charge
C) Molecular weight and isoionic pH
D) Isoionic pH and shape
E) Isoionic pH and net charge

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
A separation of a mixture of cations of different charge requires
A) another cationic substance
B) an anionic substance
C) an electrically neutral, but highly polar, substance
D) an electrically neutral, nonpolar substance
A) another cationic substance
B) an anionic substance
C) an electrically neutral, but highly polar, substance
D) an electrically neutral, nonpolar substance

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
When electrophoretic separations are done based on molecular weight,the distance that a molecule moves can be graphed as a straight line when compared to:
A) the MW of the proteins
B) the negative of the MW of the proteins
C) the log of the MW of the proteins
D) none of these
A) the MW of the proteins
B) the negative of the MW of the proteins
C) the log of the MW of the proteins
D) none of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
The most efficient method for determining the sequence of a short peptide is:
A) Edman degradation
B) Trypsin digestion
C) Chymotrypsin digestion
D) Cyanogen bromide digestion
A) Edman degradation
B) Trypsin digestion
C) Chymotrypsin digestion
D) Cyanogen bromide digestion

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
If a protein with the sequence PQRKYPIG is treated with trypsin,what will the products be?
A) PQR KYPIG
B) PQRK YPIG
C) PQR K YPIG
D) PQ R KPIG0
A) PQR KYPIG
B) PQRK YPIG
C) PQR K YPIG
D) PQ R KPIG0

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
Methods for breaking proteins into smaller peptides include all of the following except:
A) Digestion with chymotrypsin
B) Cyanogen Bromide treatment
C) Digestion with Trypsin
D) Edmann degradation
E) All of the above create short peptides suitable for sequencing
A) Digestion with chymotrypsin
B) Cyanogen Bromide treatment
C) Digestion with Trypsin
D) Edmann degradation
E) All of the above create short peptides suitable for sequencing

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
The isoelectric point is
A) the pH at which a substance has no net charge
B) the pH at which a substance has a net positive charge
C) the pH at which a substance has a net negative charge
D) the pH at which a substance has no charge groups of any kind
A) the pH at which a substance has no net charge
B) the pH at which a substance has a net positive charge
C) the pH at which a substance has a net negative charge
D) the pH at which a substance has no charge groups of any kind

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
Matrix-Assisted Laser Desorption Ionization is a type of _______ technique.
A) electrophoresis
B) ion exchange chromatography
C) affinity chromatography
D) mass spectrometry
A) electrophoresis
B) ion exchange chromatography
C) affinity chromatography
D) mass spectrometry

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following treatments results in a polypeptide fragment with a homoserine lactone at the C-terminal end?
A) trypsin
B) chymotrypsin
C) cyanogen bromide
D) Edman method
A) trypsin
B) chymotrypsin
C) cyanogen bromide
D) Edman method

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
When end-group analysis was done on the protein insulin,the results indicated that both glycine and phenylalanine were N-terminal amino acids and both asparagine and alanine were C-terminal amino acids.These results indicate that
A) the experiment was done incorrectly
B) no conclusions can be drawn
C) there were impurities in the sample
D) insulin consists of two polypeptide chains
A) the experiment was done incorrectly
B) no conclusions can be drawn
C) there were impurities in the sample
D) insulin consists of two polypeptide chains

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is not used in protein structure determination?
A) digestion with proteolytic enzymes
B) the Edman method
C) treatment with cyanogen bromide
D) treatment with alkyl halides
A) digestion with proteolytic enzymes
B) the Edman method
C) treatment with cyanogen bromide
D) treatment with alkyl halides

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
Cyanogen bromide (CNBr) cleaves proteins
A) after positively charged residues, such as K & R.
B) after negatively charged residues, such as D &
C) after aromatic residues, such as Y & W.
D) after methionine residues.
E)
A) after positively charged residues, such as K & R.
B) after negatively charged residues, such as D &
C) after aromatic residues, such as Y & W.
D) after methionine residues.
E)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
In isoelectric focusing gel electrophoresis
A) particular care must be taken to ensure the same pH along the length of the gel
B) there is a pH gradient that parallels the electric field gradient
C) the electric current is allowed to fluctuate
D) the electric circuits of the apparatus must be very well insulated
A) particular care must be taken to ensure the same pH along the length of the gel
B) there is a pH gradient that parallels the electric field gradient
C) the electric current is allowed to fluctuate
D) the electric circuits of the apparatus must be very well insulated

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
It is frequently possible to bypass the determination of the identity of the N-terminal amino acid of a protein because
A) this information is already available from the amino acid analysis
B) the Edman method sequences the peptide from the N-terminal end
C) N-terminal amino acids are always chemically modified
D) this information is not needed
A) this information is already available from the amino acid analysis
B) the Edman method sequences the peptide from the N-terminal end
C) N-terminal amino acids are always chemically modified
D) this information is not needed

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
If a protein with the sequence FEWPRQVDMARINE is treated with chymotrypsin,what will the products be?
A) F EW PRQVMARINE
B) FE WPRQVD MARINE
C) FEWPR QVDMAR INE
D) FEWPRQVDM ARINE
A) F EW PRQVMARINE
B) FE WPRQVD MARINE
C) FEWPR QVDMAR INE
D) FEWPRQVDM ARINE

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
An amino acid analyzer is an instrument used to determine
A) the sequence of amino acids in a polypeptide chain
B) the identity of N-terminal and C-terminal amino acids in a protein
C) the presence of modified amino acids in a protein
D) the identities and relative amounts of amino acids in a protein
A) the sequence of amino acids in a polypeptide chain
B) the identity of N-terminal and C-terminal amino acids in a protein
C) the presence of modified amino acids in a protein
D) the identities and relative amounts of amino acids in a protein

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
Two dimensional separation methods
A) lead to unreliable results
B) are not widely used because of their complexity
C) do not improve separation
D) consist of two separation methods applied in sequence
A) lead to unreliable results
B) are not widely used because of their complexity
C) do not improve separation
D) consist of two separation methods applied in sequence

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
Generally speaking,sequence techniques have become so sensitive that if you are able to isolate the protein on a gel,there is enough of it to get a significant amount of its sequence.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
Important steps in sequencing pure proteins include all of these except:
A) Determining the amino acid composition
B) Determining the isoionic pH of the protein
C) Breaking the protein into smaller peptides
D) Determining the amino acids on the ends of the protein
E) Determining the amino acids on the ends of the smaller peptides
A) Determining the amino acid composition
B) Determining the isoionic pH of the protein
C) Breaking the protein into smaller peptides
D) Determining the amino acids on the ends of the protein
E) Determining the amino acids on the ends of the smaller peptides

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
Determination of the sequence of amino acids in a peptide is done by
A) x-ray crystallography
B) Edman degradation
C) treatment with cyanogen bromide
D) trypsin hydrolysis
A) x-ray crystallography
B) Edman degradation
C) treatment with cyanogen bromide
D) trypsin hydrolysis

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
It is impossible to sequence a protein if you do not have overlapping sequences to work with.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


