Deck 10: Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/147
Play
Full screen (f)
Deck 10: Thermochemistry
1
Define potential energy.
A) energy associated with the temperature of an object
B) energy associated with the motion of an object
C) energy associated with the force of an object
D) energy associated with the gravity of an object
E) energy associated with the position or composition of an object
A) energy associated with the temperature of an object
B) energy associated with the motion of an object
C) energy associated with the force of an object
D) energy associated with the gravity of an object
E) energy associated with the position or composition of an object
energy associated with the position or composition of an object
2
The temperature rises from 25.00°C to 29.00°C in a bomb calorimeter when 3.50 g of sucrose undergoes combustion in a bomb calorimeter.Calculate ΔErxn for the combustion of sucrose in kJ/mol sucrose.The heat capacity of the calorimeter is 4.90 kJ/°C.The molar mass of sugar is 342.3 g/mol.
A) (- 1.92) × 103 kJ/mole
B) 1.92 × 103 kJ/mole
C) (- 1.23) × 103 kJ/mole
D) 2.35 × 104 kJ/mole
A) (- 1.92) × 103 kJ/mole
B) 1.92 × 103 kJ/mole
C) (- 1.23) × 103 kJ/mole
D) 2.35 × 104 kJ/mole
(- 1.92) × 103 kJ/mole
3
The law of ________ states that energy that can be neither created or destroyed.
A) kinetic energy
B) the consecration of energy
C) potential energy
D) the conservation of energy
E) thermochemistry
A) kinetic energy
B) the consecration of energy
C) potential energy
D) the conservation of energy
E) thermochemistry
the conservation of energy
4
A sample of copper absorbs 43.6 kJ of heat,resulting in a temperature rise of 75.0°C,determine the mass (in kg)of the copper sample if the specific heat capacity of copper is 0.385 J/g°C.
A) 1.51 kg
B) 6.62 kg
C) 1.26 kg
D) 7.94 kg
E) 3.64 kg
A) 1.51 kg
B) 6.62 kg
C) 1.26 kg
D) 7.94 kg
E) 3.64 kg

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
5
Define kinetic energy.
A) energy associated with the temperature of an object
B) energy associated with the motion of an object
C) energy associated with the force of an object
D) energy associated with the gravity of an object
E) energy associated with the position or composition of an object
A) energy associated with the temperature of an object
B) energy associated with the motion of an object
C) energy associated with the force of an object
D) energy associated with the gravity of an object
E) energy associated with the position or composition of an object

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
6
A 4.98 g sample of aniline (C6H5NH2,molar mass = 93.13 g/mol)was combusted in a bomb calorimeter with a heat capacity of 4.25 kJ/°C.If the temperature rose from 29.5°C to 69.8°C,determine the value of ΔH°comb for aniline.
A) +7.81 × 103 kJ/mol
B) -3.20 × 103 kJ/mol
C) +1.71 × 103 kJ/mol
D) -1.71 × 103 kJ/mol
E) -7.81 × 103 kJ/mol
A) +7.81 × 103 kJ/mol
B) -3.20 × 103 kJ/mol
C) +1.71 × 103 kJ/mol
D) -1.71 × 103 kJ/mol
E) -7.81 × 103 kJ/mol

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the change in internal energy (ΔE)for a system that is giving off 25.0 kJ of heat and is changing from 18.00 L to 15.00 L in volume at 1.50 atm pressure.(Remember that 101.3 J = 1 L∙atm)
A) +25.5 kJ
B) -16.0 kJ
C) -25.5 kJ
D) -24.5 kJ
E) 456 kJ
A) +25.5 kJ
B) -16.0 kJ
C) -25.5 kJ
D) -24.5 kJ
E) 456 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about energy is FALSE?
A) Energy can be converted from one type to another.
B) The total energy of a system remains constant.
C) Kinetic energy is the energy associated with its position or composition.
D) Energy is the capacity to do work.
E) Systems tend to change in order to lower their potential energy.
A) Energy can be converted from one type to another.
B) The total energy of a system remains constant.
C) Kinetic energy is the energy associated with its position or composition.
D) Energy is the capacity to do work.
E) Systems tend to change in order to lower their potential energy.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
9
For ?Esys to always be -,what must be TRUE?
A) q = w
B) +q > -w
C) +w > -q
D) -w > +q
A) q = w
B) +q > -w
C) +w > -q
D) -w > +q

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
10
A 21.8 g sample of ethanol (C2H5OH)is burned in a bomb calorimeter,according to the following reaction.If the temperature rises from 25.0 to 62.3°C,determine the heat capacity of the calorimeter.The molar mass of ethanol is 46.07 g/mol. C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = -1235 kJ
A) 4.99 kJ/°C
B) 5.65 kJ/°C
C) 63.7 kJ/°C
D) 33.1 kJ/°C
E) 15.7 kJ/°C
A) 4.99 kJ/°C
B) 5.65 kJ/°C
C) 63.7 kJ/°C
D) 33.1 kJ/°C
E) 15.7 kJ/°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
11
A 35.6 g sample of ethanol (C2H5OH)is burned in a bomb calorimeter,according to the following reaction.If the temperature rose from 35.0 to 76.0°C and the heat capacity of the calorimeter is 23.3 kJ/°C,what is the value of DH°rxn? The molar mass of ethanol is 46.07 g/mol.
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = ?
A) -1.24 × 103 kJ/mol
B) +1.24 × 103 kJ/mol
C) -8.09 × 103 kJ/mol
D) -9.55 × 103 kJ/mol
E) +9.55 × 103 kJ/mol
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = ?
A) -1.24 × 103 kJ/mol
B) +1.24 × 103 kJ/mol
C) -8.09 × 103 kJ/mol
D) -9.55 × 103 kJ/mol
E) +9.55 × 103 kJ/mol

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
12
Define thermal energy.
A) energy associated with the temperature of an object
B) energy associated with the motion of an object
C) energy associated with the force of an object
D) energy associated with the gravity of an object
E) energy associated with the position or composition of an object
A) energy associated with the temperature of an object
B) energy associated with the motion of an object
C) energy associated with the force of an object
D) energy associated with the gravity of an object
E) energy associated with the position or composition of an object

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
13
A 12.8 g sample of ethanol (C2H5OH)is burned in a bomb calorimeter with a heat capacity of 5.65 kJ/°C.Using the information below,determine the final temperature of the calorimeter if the initial temperature is 25.0°C.The molar mass of ethanol is 46.07 g/mol. C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = -1235 kJ
A) 53.4°C
B) 28.1°C
C) 111°C
D) 85.7°C
E) 74.2°C
A) 53.4°C
B) 28.1°C
C) 111°C
D) 85.7°C
E) 74.2°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
14
Determine the final temperature of a gold nugget (mass = 376 g)that starts at 398 K and loses 4.85 kJ of heat to a snowbank when it is lost.The specific heat capacity of gold is 0.128 J/g°C.
A) 133 K
B) 398 K
C) 187 K
D) 297 K
E) 377 K
A) 133 K
B) 398 K
C) 187 K
D) 297 K
E) 377 K

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following signs on q and w represent a system that is doing work on the surroundings,as well as gaining heat from the surroundings?
A) q = +, w = -
B) q = -, w = +
C) q = +, w = +
D) q = -, w = -
E) None of these represent the system referenced above.
A) q = +, w = -
B) q = -, w = +
C) q = +, w = +
D) q = -, w = -
E) None of these represent the system referenced above.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
16
A 6.55 g sample of aniline (C6H5NH2,molar mass = 93.13 g/mol)was combusted in a bomb calorimeter.If the temperature rose by 32.9°C,use the information below to determine the heat capacity of the calorimeter. 4 C6H5NH2(l)+ 35 O2(g)→ 24 CO2(g)+ 14 H2O(g)+ 4 NO2(g)
ΔH°rxn = -1.28 × 104 kJ
A) 97.3 kJ/°C
B) 38.9 kJ/°C
C) 5.94 kJ/°C
D) 6.84 kJ/°C
E) 12.8 kJ/°C
ΔH°rxn = -1.28 × 104 kJ
A) 97.3 kJ/°C
B) 38.9 kJ/°C
C) 5.94 kJ/°C
D) 6.84 kJ/°C
E) 12.8 kJ/°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
17
Energy that is associated with the relative positions of electrons and nuclei in atoms and molecules is called
A) kinetic energy.
B) thermal energy.
C) gravitational energy.
D) chemical energy.
A) kinetic energy.
B) thermal energy.
C) gravitational energy.
D) chemical energy.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
18
Determine the specific heat capacity of an alloy that requires 59.3 kJ to raise the temperature of 150.0 g alloy from 298 K to 398 K.
A) 4.38 J/g°C
B) 2.29 J/g°C
C) 3.95 J/g°C
D) 2.53 J/g°C
E) 1.87 J/g°C
A) 4.38 J/g°C
B) 2.29 J/g°C
C) 3.95 J/g°C
D) 2.53 J/g°C
E) 1.87 J/g°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the change in internal energy (ΔE)for a system that is giving off 25.0 kJ of heat and is changing from 12.00 L to 6.00 L in volume at 1.50 atm pressure.(Remember that 101.3 J = 1 L∙atm)
A) +25.9 kJ
B) -16.0 kJ
C) -25.9 kJ
D) -24.1 kJ
E) 937 kJ
A) +25.9 kJ
B) -16.0 kJ
C) -25.9 kJ
D) -24.1 kJ
E) 937 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following (with specific heat capacity provided)would show the smallest temperature change upon gaining 200.0 J of heat?
A) 50.0 g Al, CAl = 0.903 J/g°C
B) 50.0 g Cu, CCu = 0.385 J/g°C
C) 25.0 g granite, Cgranite = 0.79 J/g°C
D) 25.0 g Au, CAu = 0.128 J/g°C
E) 25.0 g Ag, CAg = 0.235 J/g°C
A) 50.0 g Al, CAl = 0.903 J/g°C
B) 50.0 g Cu, CCu = 0.385 J/g°C
C) 25.0 g granite, Cgranite = 0.79 J/g°C
D) 25.0 g Au, CAu = 0.128 J/g°C
E) 25.0 g Ag, CAg = 0.235 J/g°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
21
According to the following reaction,how much energy is evolved during the reaction of 0.111 mol B2H6 and 0.252 mol Cl2 (Both gases are initially at STP)? The molar mass of B2H6 is 27.67 g/mol.
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A) 58.7 kJ
B) 156 kJ
C) 215 kJ
D) 352 kJ
E) 508 kJ
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A) 58.7 kJ
B) 156 kJ
C) 215 kJ
D) 352 kJ
E) 508 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
22
A student is preparing to perform a series of calorimetry experiments.She first wishes to determine the calorimeter constant (Ccal)for her coffee cup calorimeter.She pours a 50.0 mL sample of water at 345 K into the calorimeter containing a 50.0 mL sample of water at 298 K.She carefully records the final temperature of the water as 317 K.What is the value of Ccal for the calorimeter?
A) 19 J/K
B) 28 J/K
C) 99 J/K
D) 21 J/K
E) 76 J/K
A) 19 J/K
B) 28 J/K
C) 99 J/K
D) 21 J/K
E) 76 J/K

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is TRUE?
A) State functions do not depend on the path taken to arrive at a particular state.
B) DErxn can be determined using constant volume calorimetry.
C) Energy is neither created nor destroyed, excluding nuclear reactions.
D) ΔHrxn can be determined using constant pressure calorimetry.
E) All of the above are true.
A) State functions do not depend on the path taken to arrive at a particular state.
B) DErxn can be determined using constant volume calorimetry.
C) Energy is neither created nor destroyed, excluding nuclear reactions.
D) ΔHrxn can be determined using constant pressure calorimetry.
E) All of the above are true.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
24
According to the following reaction,how much energy is evolved during the reaction of 32.5 g B2H6 and 72.5 g Cl2? The molar mass of B2H6 is 27.67 g/mol.
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A) 1640 kJ
B) 238 kJ
C) 1430 kJ
D) 3070 kJ
E) 429 kJ
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A) 1640 kJ
B) 238 kJ
C) 1430 kJ
D) 3070 kJ
E) 429 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
25
How much energy is evolved during the formation of 197 g of Fe,according to the reaction below?
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A) 1.52 × 103 kJ
B) 3.02 × 103 kJ
C) 8.40 × 103 kJ
D) 964 kJ
E) 482 kJ
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A) 1.52 × 103 kJ
B) 3.02 × 103 kJ
C) 8.40 × 103 kJ
D) 964 kJ
E) 482 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
26
A 6.55 g sample of aniline (C6H5NH2,molar mass = 93.13 g/mol)was combusted in a bomb calorimeter with a heat capacity of 14.25 kJ/°C.If the initial temperature was 32.9°C,use the information below to determine the value of the final temperature of the calorimeter. 4 C6H5NH2(l)+ 35 O2(g)→ 24 CO2(g)+ 14 H2O(g)+ 4 NO2(g)
ΔH°rxn = -1.28 × 104 kJ
A) 257°C
B) 46.6°C
C) 48.7°C
D) 41.9°C
E) 931°C
ΔH°rxn = -1.28 × 104 kJ
A) 257°C
B) 46.6°C
C) 48.7°C
D) 41.9°C
E) 931°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
27
Which statement is FALSE?
A) An exothermic reaction gives heat off heat to the surroundings.
B) Enthalpy is the sum of a system's internal energy and the product of pressure and volume change.
C) ΔErxn is a measure of heat.
D) ΔHrxn is the heat of reaction.
E) Endothermic has a positive ΔH.
A) An exothermic reaction gives heat off heat to the surroundings.
B) Enthalpy is the sum of a system's internal energy and the product of pressure and volume change.
C) ΔErxn is a measure of heat.
D) ΔHrxn is the heat of reaction.
E) Endothermic has a positive ΔH.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
28
A piece of iron (mass = 25.0 g)at 398 K is placed in a styrofoam coffee cup containing 25.0 mL of water at 298 K.Assuming that no heat is lost to the cup or the surroundings,what will the final temperature of the water be? The specific heat capacity of iron = 0.449 J/g°C and water = 4.18 J/g°C.
A) 348 K
B) 308 K
C) 287 K
D) 325 K
E) 388 K
A) 348 K
B) 308 K
C) 287 K
D) 325 K
E) 388 K

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
29
Using the following equation for the combustion of octane,calculate the amount of moles of oxygen that reacts with 100.0 g of octane.The molar mass of octane is 114.33 g/mole.The molar mass of carbon dioxide is 44.0095 g/mole. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH°rxn = -11018 kJ
A) 18.18 moles
B) 6.997 moles
C) 14.00 moles
D) 8.000 moles
E) 10.93 moles
A) 18.18 moles
B) 6.997 moles
C) 14.00 moles
D) 8.000 moles
E) 10.93 moles

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
30
Given w = 0,an endothermic reaction has the following.
A) +ΔH and -ΔE
B) - ΔH and +ΔE
C) + ΔH and +ΔE
D) - ΔH and -ΔE
A) +ΔH and -ΔE
B) - ΔH and +ΔE
C) + ΔH and +ΔE
D) - ΔH and -ΔE

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
31
Using the following equation for the combustion of octane,calculate the amount of grams of carbon dioxide formed from 100.0 g of octane.The molar mass of octane is 114.33 g/mole.The molar mass of carbon dioxide is 44.0095 g/mole. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH°rxn = -11018 kJ
A) 800.1 g
B) 307.9 g
C) 260.1 g
D) 792.3 g
A) 800.1 g
B) 307.9 g
C) 260.1 g
D) 792.3 g

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
32
How much energy is required to decompose 765 g of PCl3,according to the reaction below? The molar mass of PCl3 is 137.32 g/mol and may be useful.
4 PCl3(g)→ P4(s)+ 6 Cl2(g)ΔH°rxn = +1207 kJ
A) 2.31 × 103 kJ
B) 4.33 × 103 kJ
C) 6.72 × 103 kJ
D) 1.68 × 103 kJ
E) 5.95 × 103 kJ
4 PCl3(g)→ P4(s)+ 6 Cl2(g)ΔH°rxn = +1207 kJ
A) 2.31 × 103 kJ
B) 4.33 × 103 kJ
C) 6.72 × 103 kJ
D) 1.68 × 103 kJ
E) 5.95 × 103 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
33
Using the following thermochemical equation,determine the amount of heat produced per kg of CO2 formed during the combustion of benzene (C6H6). 2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A) 1.43 × 105 kJ/kg CO2
B) 2.30 × 104 kJ/kg CO2
C) 4.34 × 104 kJ/kg CO2
D) 1.19 × 104 kJ/kg CO2
E) 8.40 × 105 kJ/kg CO2
A) 1.43 × 105 kJ/kg CO2
B) 2.30 × 104 kJ/kg CO2
C) 4.34 × 104 kJ/kg CO2
D) 1.19 × 104 kJ/kg CO2
E) 8.40 × 105 kJ/kg CO2

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
34
A 100.0 mL sample of 0.300 M NaOH is mixed with a 100.0 mL sample of 0.300 M HNO3 in a coffee cup calorimeter.If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C,determine the ΔH°rxn (in units of kJ/mol NaOH)for the neutralization reaction between aqueous NaOH and HCl.Assume 1)that no heat is lost to the calorimeter or the surroundings,and 2)that the density and the heat capacity of the resulting solution are the same as water.
A) -55.7 kJ/mol NaOH
B) -169 kJ/mol NaOH
C) -16.7 kJ/mol NaOH
D) -27.9 kJ/mol NaOH
E) - 34.4 kJ/mol NaOH
A) -55.7 kJ/mol NaOH
B) -169 kJ/mol NaOH
C) -16.7 kJ/mol NaOH
D) -27.9 kJ/mol NaOH
E) - 34.4 kJ/mol NaOH

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
35
Using the following equation for the combustion of octane,calculate the amount of moles of carbon dioxide formed from 100.0 g of octane.The molar mass of octane is 114.33 g/mole.The molar mass of carbon dioxide is 44.0095 g/mole. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH°rxn = -11018 kJ
A) 18.18 moles
B) 6.997 moles
C) 14.00 moles
D) 8.000 moles
E) 10.93 moles
A) 18.18 moles
B) 6.997 moles
C) 14.00 moles
D) 8.000 moles
E) 10.93 moles

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
36
How much energy is required to decompose 612 g of PCl3,according to the reaction below? The molar mass of PCl3 is 137.32 g/mol and may be useful.
4 PCl3(g)→ P4(s)+ 6 Cl2(g)ΔH°rxn = +1207 kJ
A) 1.85 × 103 kJ
B) 3.46 × 103 kJ
C) 5.38 × 103 kJ
D) 1.34 × 103 kJ
E) 4.76 × 103 kJ
4 PCl3(g)→ P4(s)+ 6 Cl2(g)ΔH°rxn = +1207 kJ
A) 1.85 × 103 kJ
B) 3.46 × 103 kJ
C) 5.38 × 103 kJ
D) 1.34 × 103 kJ
E) 4.76 × 103 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
37
How much energy is evolved during the formation of 98.7 g of Fe,according to the reaction below?
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A) 753 kJ
B) 1.51 × 103 kJ
C) 4.20 × 103 kJ
D) 482 kJ
E) 241 kJ
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A) 753 kJ
B) 1.51 × 103 kJ
C) 4.20 × 103 kJ
D) 482 kJ
E) 241 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
38
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter.The reaction causes the temperature of the resulting solution to fall below room temperature.Which of the following statements is TRUE?
A) The products have a lower potential energy than the reactants.
B) This type of experiment will provide data to calculate ΔErxn.
C) The reaction is exothermic.
D) Energy is leaving the system during reaction.
E) None of the above statements are true.
A) The products have a lower potential energy than the reactants.
B) This type of experiment will provide data to calculate ΔErxn.
C) The reaction is exothermic.
D) Energy is leaving the system during reaction.
E) None of the above statements are true.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
39
According to the following thermochemical equation,what mass of HF (in g)must react in order to produce 345 kJ of energy? Assume excess SiO2.
SiO2(s)+ 4 HF(g)→ SiF4(g)+ 2 H2O(l)ΔH°rxn = -184 kJ
A) 42.7 g
B) 37.5 g
C) 150. g
D) 107 g
E) 173 g
SiO2(s)+ 4 HF(g)→ SiF4(g)+ 2 H2O(l)ΔH°rxn = -184 kJ
A) 42.7 g
B) 37.5 g
C) 150. g
D) 107 g
E) 173 g

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
40
What volume of benzene (C6H6,d= 0.88 g/mL,molar mass = 78.11 g/mol)is required to produce 1.5 × 103 kJ of heat according to the following reaction?
2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A) 75 mL
B) 37 mL
C) 21 mL
D) 19 mL
E) 42 mL
2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A) 75 mL
B) 37 mL
C) 21 mL
D) 19 mL
E) 42 mL

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following processes are endothermic?
A) the reaction associated with the lattice energy of LiCl
B) the reaction associated with the ionization energy of potassium
C) the reaction associated with the heat of formation of CaS
D) the formation of F2 from its elements in their standard states
E) None of the above are endothermic.
A) the reaction associated with the lattice energy of LiCl
B) the reaction associated with the ionization energy of potassium
C) the reaction associated with the heat of formation of CaS
D) the formation of F2 from its elements in their standard states
E) None of the above are endothermic.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
42
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction 2 NO(g)+ O2(g)→ 2 NO2(g)ΔH°rxn = ?
Given:
N2(g)+ O2(g)→ 2 NO(g)ΔH°rxn = +183 kJ
1/2 N2(g)+ O2(g)→ NO2(g)ΔH°rxn = +33 kJ
A) -150. kJ
B) -117 kJ
C) -333 kJ
D) +115 kJ
E) +238 kJ
Given:
N2(g)+ O2(g)→ 2 NO(g)ΔH°rxn = +183 kJ
1/2 N2(g)+ O2(g)→ NO2(g)ΔH°rxn = +33 kJ
A) -150. kJ
B) -117 kJ
C) -333 kJ
D) +115 kJ
E) +238 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
43
Use the bond energies provided to estimate ΔH°rxn for the reaction below. XeF2 + 2 F2 → XeF6 ΔH°rxn = ?
Bond Bond Energy (kJ/mol)
Xe-F 147
F-F 159
A) -429 kJ
B) +159 kJ
C) -660 kJ
D) +176 kJ
E) -270 kJ
Bond Bond Energy (kJ/mol)
Xe-F 147
F-F 159
A) -429 kJ
B) +159 kJ
C) -660 kJ
D) +176 kJ
E) -270 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
44
Use the bond energies provided to estimate ΔH°rxn for the reaction below. 2 Br2(l)+ C2H2(g)→ C2H2Br4(l)ΔH°rxn = ?
Bond Bond Energy (kJ/mol)
Br-Br 193
C≡C 837
C-C 347
C-Br 276
C-H 414
A) +407 kJ
B) -324 kJ
C) -228 kJ
D) +573 kJ
E) -648 kJ
Bond Bond Energy (kJ/mol)
Br-Br 193
C≡C 837
C-C 347
C-Br 276
C-H 414
A) +407 kJ
B) -324 kJ
C) -228 kJ
D) +573 kJ
E) -648 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following processes are endothermic?
A) K⁺(g) + I⁻(g) → KI(s)
B) 2 Br(g) → Br2(g)
C) Ca(s) → Ca(g)
D) 2 Na(s) +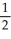 O2(g) → Na2O(s)
O2(g) → Na2O(s)
E) None of the above are endothermic.
A) K⁺(g) + I⁻(g) → KI(s)
B) 2 Br(g) → Br2(g)
C) Ca(s) → Ca(g)
D) 2 Na(s) +
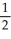 O2(g) → Na2O(s)
O2(g) → Na2O(s)E) None of the above are endothermic.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
46
Two solutions,initially at 24.69°C,are mixed in a coffee cup calorimeter (Ccal = 105.5 J/°C).When a 200.0 mL volume of 0.100 M AgNO3 solution is mixed with a 100.0 mL sample of 0.100 M NaCl solution,the temperature in the calorimeter rises to 25.16°C.Determine the DH°rxn,in units of kJ/mol AgCl.Assume that the density and heat capacity of the solutions is the same as that of water.Hint: Write a balanced reaction for the process.
A) -32 kJ/mol AgCl
B) -78 kJ/mol AgCl
C) -64 kJ/mol AgCl
D) -25 kJ/mol AgCl
E) -59 kJ/mol AgCl
A) -32 kJ/mol AgCl
B) -78 kJ/mol AgCl
C) -64 kJ/mol AgCl
D) -25 kJ/mol AgCl
E) -59 kJ/mol AgCl

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
47
Use the bond energies provided to estimate ΔH°rxn for the reaction below. C2H4(g)+ H2(g)→ C2H6(g)ΔH°rxn = ?
Bond Bond Energy (kJ/mol)
C-C 347
C-H 414
C=C 611
C≡C 837
H-H 436
A) -128 kJ
B) +98 kJ
C) +700 kJ
D) -102 kJ
E) -166 kJ
Bond Bond Energy (kJ/mol)
C-C 347
C-H 414
C=C 611
C≡C 837
H-H 436
A) -128 kJ
B) +98 kJ
C) +700 kJ
D) -102 kJ
E) -166 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
48
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction 2 S(s)+ 3 O2(g)→ 2 SO3(g)ΔH°rxn = ?
Given:
SO2(g)→ S(s)+ O2(g)ΔH°rxn = +296.8 kJ
2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -197.8 kJ
A) -494.6 kJ
B) -692.4 kJ
C) -791.4 kJ
D) 1583 kJ
E) -293.0 kJ
Given:
SO2(g)→ S(s)+ O2(g)ΔH°rxn = +296.8 kJ
2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -197.8 kJ
A) -494.6 kJ
B) -692.4 kJ
C) -791.4 kJ
D) 1583 kJ
E) -293.0 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following processes are exothermic?
A) the second ionization energy of Mg
B) the sublimation of Li
C) the breaking the bond of I2
D) the formation of NaBr from its constituent elements in their standard state
E) None of the above are exothermic.
A) the second ionization energy of Mg
B) the sublimation of Li
C) the breaking the bond of I2
D) the formation of NaBr from its constituent elements in their standard state
E) None of the above are exothermic.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following processes are exothermic?
A) Cl2(g) → 2Cl(g)
B) Br(g) + e⁻ → Br⁻(g)
C) Li(s) → Li(g)
D) NaF(s) → Na⁺(g) + F⁻(g)
E) None of the above are exothermic.
A) Cl2(g) → 2Cl(g)
B) Br(g) + e⁻ → Br⁻(g)
C) Li(s) → Li(g)
D) NaF(s) → Na⁺(g) + F⁻(g)
E) None of the above are exothermic.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
51
Use the ΔH°f information provided to calculate ΔH°rxn for the following ΔH°f (kJ/mol) SO2Cl2(g)+ 2 H2O(l)→ 2 HCl(g)+ H2SO4(l)ΔH°rxn = ?
SO2Cl2(g)-364
H2O(l)-286
HCl(g)-92
H2SO4(l)-814
A) -256 kJ
B) +161 kJ
C) -62 kJ
D) +800. kJ
E) -422 kJ
SO2Cl2(g)-364
H2O(l)-286
HCl(g)-92
H2SO4(l)-814
A) -256 kJ
B) +161 kJ
C) -62 kJ
D) +800. kJ
E) -422 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
52
Use the ΔH°f and ΔH°rxn information provided to calculate ΔH°f for IF ΔH°f (kJ/mol) IF7(g)+ I2(g)→ IF5(g)+ 2 IF(g)ΔH°rxn = -89 kJ
IF7(g)941
IF5(g)840
A) 101 kJ/mol
B) -146 kJ/mol
C) -190. kJ/mol
D) -95 kJ/mol
E) 24 kJ/mol
IF7(g)941
IF5(g)840
A) 101 kJ/mol
B) -146 kJ/mol
C) -190. kJ/mol
D) -95 kJ/mol
E) 24 kJ/mol

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
53
Use the bond energies provided to estimate ΔH°rxn for the reaction below. CH3OH(l)+ 2 O2(g)→ CO2(g)+ 2 H2O(g)ΔH°rxn = ?
Bond Bond Energy (kJ/mol)
C-H 414
C-O 360
C=O 799
O=O 498
O-H 464
A) +473 kJ
B) -91 kJ
C) -486 kJ
D) -392 kJ
E) +206 kJ
Bond Bond Energy (kJ/mol)
C-H 414
C-O 360
C=O 799
O=O 498
O-H 464
A) +473 kJ
B) -91 kJ
C) -486 kJ
D) -392 kJ
E) +206 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
54
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction P4(g)+ 10 Cl2(g)→ 4PCl5(s)ΔH°rxn = ?
Given:
PCl5(s)→ PCl3(g)+ Cl2(g)ΔH°rxn = +157 kJ
P4(g)+ 6 Cl2(g)→ 4 PCl3(g)ΔH°rxn = -1207 kJ
A) -1835 kJ
B) -1364 kJ
C) -1050. kJ
D) -1786 kJ
E) -2100. kJ
Given:
PCl5(s)→ PCl3(g)+ Cl2(g)ΔH°rxn = +157 kJ
P4(g)+ 6 Cl2(g)→ 4 PCl3(g)ΔH°rxn = -1207 kJ
A) -1835 kJ
B) -1364 kJ
C) -1050. kJ
D) -1786 kJ
E) -2100. kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is NOT a standard state?
A) For a solid, it is 25°F.
B) For a liquid, it is 25°C.
C) For a gas, it is 1 atm.
D) For a solution, it is 1 M.
E) For a liquid, it is 1 atm.
A) For a solid, it is 25°F.
B) For a liquid, it is 25°C.
C) For a gas, it is 1 atm.
D) For a solution, it is 1 M.
E) For a liquid, it is 1 atm.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
56
Choose the thermochemical equation that illustrates ΔH°f for Li2SO4.
A) 2 Li+(aq) + SO42-(aq) → Li2SO4(aq)
B) 2 Li(s) + 1/8 S8(s, rhombic) + 2 O2(g) → Li2SO4(s)
C) Li2SO4(aq) → 2 Li+(aq) + SO42-(aq)
D) 8 Li2SO4(s) → 16 Li(s) + S8(s, rhombic) + 16 O2(g)
E) 16 Li(s) + S8(s, rhombic) + 16 O2(g) → 8 Li2SO4(s)
A) 2 Li+(aq) + SO42-(aq) → Li2SO4(aq)
B) 2 Li(s) + 1/8 S8(s, rhombic) + 2 O2(g) → Li2SO4(s)
C) Li2SO4(aq) → 2 Li+(aq) + SO42-(aq)
D) 8 Li2SO4(s) → 16 Li(s) + S8(s, rhombic) + 16 O2(g)
E) 16 Li(s) + S8(s, rhombic) + 16 O2(g) → 8 Li2SO4(s)

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
57
Use the bond energies provided to estimate ΔH°rxn for the reaction below. PCl3(g)+ Cl2(g)→ PCl5(l)ΔH°rxn = ?
Bond Bond Energy (kJ/mol)
Cl-Cl 243
P-Cl 331
A) -243 kJ
B) -419 kJ
C) -662 kJ
D) -67 kJ
E) -905 kJ
Bond Bond Energy (kJ/mol)
Cl-Cl 243
P-Cl 331
A) -243 kJ
B) -419 kJ
C) -662 kJ
D) -67 kJ
E) -905 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
58
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction 4 SO3(g)→ 4 S(s)+ 6 O2(g)ΔH°rxn = ?
Given:
SO2(g)→ S(s)+ O2(g)ΔH°rxn = +296.8 kJ
2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -197.8 kJ
A) -494.6 kJ
B) -692.4 kJ
C) -791.4 kJ
D) 1583 kJ
E) -293.0 kJ
Given:
SO2(g)→ S(s)+ O2(g)ΔH°rxn = +296.8 kJ
2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -197.8 kJ
A) -494.6 kJ
B) -692.4 kJ
C) -791.4 kJ
D) 1583 kJ
E) -293.0 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is NOT a standard state?
A) For a liquid, it is 25°F.
B) For a solid, it is 25°C.
C) For a solid, it is 1 atm.
D) For a solution, it is 1 M.
E) For a liquid, it is 1 atm.
A) For a liquid, it is 25°F.
B) For a solid, it is 25°C.
C) For a solid, it is 1 atm.
D) For a solution, it is 1 M.
E) For a liquid, it is 1 atm.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
60
Two solutions,initially at 24.60°C,are mixed in a coffee cup calorimeter (Ccal = 15.5 J/°C).When a 100.0 mL volume of 0.100 M AgNO3 solution is mixed with a 100.0 mL sample of 0.200 M NaCl solution,the temperature in the calorimeter rises to 25.30°C.Determine the DH°rxn for the reaction as written below.Assume that the density and heat capacity of the solutions is the same as that of water. NaCl(aq)+ AgNO3(aq)→ AgCl(s)+ NaNO3(aq)DH°rxn = ?
A) -35 kJ
B) -69 kJ
C) -250 kJ
D) -16 kJ
E) -140 kJ
A) -35 kJ
B) -69 kJ
C) -250 kJ
D) -16 kJ
E) -140 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
61
Define heat capacity.
A) the quantity of heat required to lower the temperature of 1 mole of a substance by 1°C
B) the quantity of heat required to change a system's temperature by 1°C
C) the quantity of heat required to lower the temperature of 1 gram of a substance by 1°C
D) the quantity of heat required to raise the temperature of 1 g of a substance by 1°F
E) the quantity of heat required to lower the temperature of 1 liter of a substance by 1°C
A) the quantity of heat required to lower the temperature of 1 mole of a substance by 1°C
B) the quantity of heat required to change a system's temperature by 1°C
C) the quantity of heat required to lower the temperature of 1 gram of a substance by 1°C
D) the quantity of heat required to raise the temperature of 1 g of a substance by 1°F
E) the quantity of heat required to lower the temperature of 1 liter of a substance by 1°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
62
Use the information provided to determine ΔH°rxn for the following reaction ΔH°f (kJ/mol)CH4(g)+ 3 Cl2(g)→ CHCl3(l)+ 3 HCl(g)ΔH°rxn = ?
CH4(g)-75
CHCl3(l)-134
HCl(g)-92
A) -151 kJ
B) -335 kJ
C) +662 kJ
D) +117 kJ
E) -217 kJ
CH4(g)-75
CHCl3(l)-134
HCl(g)-92
A) -151 kJ
B) -335 kJ
C) +662 kJ
D) +117 kJ
E) -217 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
63
Which compound should have the largest lattice energy?
A) SrI2
B) CaCl2
C) NaI
D) MgO
E) KF
A) SrI2
B) CaCl2
C) NaI
D) MgO
E) KF

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the kinetic energy of a 150 g baseball moving at a speed of 39.m/s ( 87 mph).
A) 5.8 J
B) 1.1 × 102 J
C) 5.8 × 103 J
D) 1.1 × 105 J
A) 5.8 J
B) 1.1 × 102 J
C) 5.8 × 103 J
D) 1.1 × 105 J

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of NI3 is contained in a piston and cylinder.The samples rapidly decomposes to form nitrogen gas and iodine gas,and releases 3.30 kJ of heat and does 950 J of work.What is ∆E?
A) -953.3 J
B) +953.3 J
C) -4250 J
D) -946.7 J
E) +4250 J
A) -953.3 J
B) +953.3 J
C) -4250 J
D) -946.7 J
E) +4250 J

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
66
Which one of the following statements is NOT true concerning the equation below?
N2(g)+ 3 H2(g)→ 2 NH3(g)ΔH°rxn = -459.0 kJ
A) The production of 1.00 mole of ammonia is accompanied by the production of 229.5 kJ of heat.
B) The complete reaction of 1.00 moles of nitrogen requires 459 kJ of heat.
C) The complete reaction of 1.00 moles of hydrogen produces 153 kJ of heat.
D) The complete reaction of 0.8278 moles of hydrogen requires 0.2759 moles of nitrogen.
E) The reaction is exothermic.
N2(g)+ 3 H2(g)→ 2 NH3(g)ΔH°rxn = -459.0 kJ
A) The production of 1.00 mole of ammonia is accompanied by the production of 229.5 kJ of heat.
B) The complete reaction of 1.00 moles of nitrogen requires 459 kJ of heat.
C) The complete reaction of 1.00 moles of hydrogen produces 153 kJ of heat.
D) The complete reaction of 0.8278 moles of hydrogen requires 0.2759 moles of nitrogen.
E) The reaction is exothermic.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
67
Use the information provided to determine ΔH°rxn for the following reaction ΔH°f (kJ/mol)3 Fe2O3(s)+ CO(g)→ 2 Fe3O4(s)+ CO2(g)ΔH°rxn = ?
Fe2O3(s)-824
Fe3O4(s)-1118
CO(g)-111
CO2(g)-394
A) +277 kJ
B) -577 kJ
C) -47 kJ
D) +144 kJ
E) -111 kJ
Fe2O3(s)-824
Fe3O4(s)-1118
CO(g)-111
CO2(g)-394
A) +277 kJ
B) -577 kJ
C) -47 kJ
D) +144 kJ
E) -111 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
68
Which one of the following elements is NOT in its standard state?
A) Br2(g)
B) F2(g)
C) Hg(l)
D) Na(s)
E) Cl2(g)
A) Br2(g)
B) F2(g)
C) Hg(l)
D) Na(s)
E) Cl2(g)

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
69
Use the information provided to determine ΔH°rxn for the following reaction ΔH°f (kJ/mol)CH4(g)+ 4 Cl2(g)→ CCl4(g)+ 4 HCl(g)ΔH°rxn = ?
CH4(g)-75
CCl4(g)-96
HCl(g)-92
A) -389 kJ
B) -113 kJ
C) +113 kJ
D) -71 kJ
E) +79 kJ
CH4(g)-75
CCl4(g)-96
HCl(g)-92
A) -389 kJ
B) -113 kJ
C) +113 kJ
D) -71 kJ
E) +79 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the change internal energy (ΔE)for a system that is giving off 65.0 kJ of heat and is performing 855 J of work on the surroundings.
A) 64.1 kJ
B) -64.1 kJ
C) -65.9 kJ
D) 9.00 × 102 kJ
E) -9.00 × 102 kJ
A) 64.1 kJ
B) -64.1 kJ
C) -65.9 kJ
D) 9.00 × 102 kJ
E) -9.00 × 102 kJ

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is TRUE if ΔEsys = - 115 J?
A) The system is gaining 115 J, while the surroundings are losing 115 J.
B) The system is losing 115 J, while the surroundings are gaining 115 J.
C) Both the system and the surroundings are gaining 115 J.
D) Both the system and the surroundings are losing 115 J.
E) None of the above are true.
A) The system is gaining 115 J, while the surroundings are losing 115 J.
B) The system is losing 115 J, while the surroundings are gaining 115 J.
C) Both the system and the surroundings are gaining 115 J.
D) Both the system and the surroundings are losing 115 J.
E) None of the above are true.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
72
For the reaction: NO(g)+ 1/2 O2(g)→ NO2(g)
ΔH°rxn is -114.14 kJ/mol.Calculate ΔH°f of gaseous nitrogen monoxide,given that ΔH°f of NO2(g)is 33.90 kJ/mol.
A) 148.0 kJ/mol
B) 91.04 kJ/mol
C) -114.1 kJ/mol
D) 181.9 kJ/mol
E) -35.64 kJ/mol
ΔH°rxn is -114.14 kJ/mol.Calculate ΔH°f of gaseous nitrogen monoxide,given that ΔH°f of NO2(g)is 33.90 kJ/mol.
A) 148.0 kJ/mol
B) 91.04 kJ/mol
C) -114.1 kJ/mol
D) 181.9 kJ/mol
E) -35.64 kJ/mol

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
73
A 43.9-g piece of copper (CCu= 0.385 J/g°C)at 135.0°C is plunged into 254 g of water at 39.0°C.Assuming that no heat is lost to the surroundings,what will the final temperature of the system be?
A) 100.0°C
B) 40.5°C
C) 62.5°C
D) 87.0°C
E) 53.1°C
A) 100.0°C
B) 40.5°C
C) 62.5°C
D) 87.0°C
E) 53.1°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
74
All the following are state functions EXCEPT
A) ∆E.
B) PV.
C) ∆H.
D) q.
E) all of the above are state functions.
A) ∆E.
B) PV.
C) ∆H.
D) q.
E) all of the above are state functions.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
75
A piece of metal weighing 57.3 g is heated to a temperature of 88.0°C and is then immersed in 155 g of water at a temperature of 21.53°C.After equilibration the temperature is 24.72°C.If CH2O = 4.184 J/g°C,what is Cmetal?
A) .370 J/g°C
B) .164 J/g°C
C) 1.00 J/g°C
D) 2.11 J/g°C
E) .571 J/g°C
A) .370 J/g°C
B) .164 J/g°C
C) 1.00 J/g°C
D) 2.11 J/g°C
E) .571 J/g°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
76
Define specific heat capacity.
A) the quantity of heat required to lower the temperature of 1 mole of a substance by 1°C
B) the quantity of heat required to change a system's temperature by 1°C
C) the quantity of heat required to raise the temperature of 1 gram of a substance by 1°C
D) the quantity of heat required to lower the temperature of 1 gram of a substance by 1°F
E) the quantity of heat required to lower the temperature of 1 liter of a substance by 1°C
A) the quantity of heat required to lower the temperature of 1 mole of a substance by 1°C
B) the quantity of heat required to change a system's temperature by 1°C
C) the quantity of heat required to raise the temperature of 1 gram of a substance by 1°C
D) the quantity of heat required to lower the temperature of 1 gram of a substance by 1°F
E) the quantity of heat required to lower the temperature of 1 liter of a substance by 1°C

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
77
In a bomb calorimeter,reactions are carried out
A) at fixed pressure.
B) at fixed volume.
C) at fixed temperature.
D) with solids only.
E) using water as a solvent.
A) at fixed pressure.
B) at fixed volume.
C) at fixed temperature.
D) with solids only.
E) using water as a solvent.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
78
Use the ΔH°f and ΔH°rxn information provided to calculate ΔH°f for SO3(g) ΔH°f (kJ/mol) 2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -198 kJ
SO2(g)-297
A) -792 kJ/mol
B) -248 kJ/mol
C) -495 kJ/mol
D) -578 kJ/mol
E) -396 kJ/mol
SO2(g)-297
A) -792 kJ/mol
B) -248 kJ/mol
C) -495 kJ/mol
D) -578 kJ/mol
E) -396 kJ/mol

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
79
How much heat energy is required to raise the temperature of 0.298 mole of water from 35.93°C to 79.84°C? The specific heat capacity of water = 4.18 J/g°C.
A) 13.1 J
B) 54.7 J
C) 985 J
D) 2105 J
E) 3310 J
A) 13.1 J
B) 54.7 J
C) 985 J
D) 2105 J
E) 3310 J

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck
80
For a process at constant pressure,49,600 calories of heat are released.This quantity of heat is equivalent to
A) 4.82 × 10-6 J.
B) 1.19 × 104 J.
C) 1.24 × 104 J.
D) 2.08 × 105 J.
A) 4.82 × 10-6 J.
B) 1.19 × 104 J.
C) 1.24 × 104 J.
D) 2.08 × 105 J.

Unlock Deck
Unlock for access to all 147 flashcards in this deck.
Unlock Deck
k this deck


