Deck 9: Activity and the Systematic Treatment of Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 9: Activity and the Systematic Treatment of Equilibrium
1
Calculate the ionic strength of a 0.210 M FeCl2 solution.
0.630
2
Which equation is NOT required to determine the pH of 0.10 M solution of weak acid,HA?
A)HA + H2O ⇋ A− + H3O+ Ka = 2.4 × 10−6
B)H2O + H2O ⇋ H3O+ + OH− Kw = 1 × 10−14
C)0)10 = [HA] + [A−]
D)[H3O+] = [OH−] + [A−]
E)A− + H2O ⇋ HA + OH− Kb = 4.2 × 10−9
A)HA + H2O ⇋ A− + H3O+ Ka = 2.4 × 10−6
B)H2O + H2O ⇋ H3O+ + OH− Kw = 1 × 10−14
C)0)10 = [HA] + [A−]
D)[H3O+] = [OH−] + [A−]
E)A− + H2O ⇋ HA + OH− Kb = 4.2 × 10−9
A− + H2O ⇋ HA + OH− Kb = 4.2 × 10−9
3
Calculate the pH of a 0.002 M (CH3)3N solution.Kb = 6.6 × 10−5
10.62
4
Calculate the pH of a 0.040 M HCl solution that is also 0.010 M in NaNO3.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
For Ag+ when = 0.01 M, = 0.898 and when = 0.05, = 0.80.What is the activity coefficient when = 0.024 M?
A)0)804
B)0)845
C)0)853
D)0)864
E)0)894
A)0)804
B)0)845
C)0)853
D)0)864
E)0)894

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
The mass balance equation for a 0.10 M HO2CCO2H solution is which of the following?
A)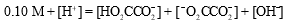
B)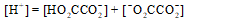
C)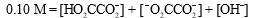
D)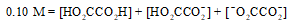
E)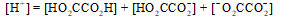
A)
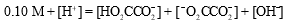
B)
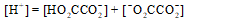
C)
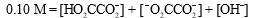
D)
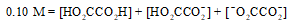
E)
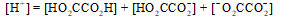

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
A buffer is prepared from NaH2PO4 and Na2HPO4.The charge balance equation for the buffer is which of the following?
A)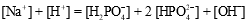
B)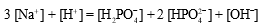
C)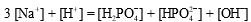
D)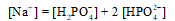
E)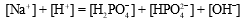
A)
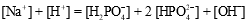
B)
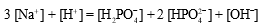
C)
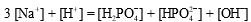
D)
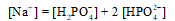
E)
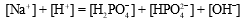

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Which equation is NOT required to determine the molar solubility of AgCN?
A)AgCN ⇋ Ag+ + CN−____________________Ksp = 2.2 × 10−16
B)H2O + H2O ⇋ H3O+ + OH− Kw = 1 × 10−14
C)[Ag+] = [CN−] + [HCN]
D)CN− + H2O ⇋ HCN + OH− Kb = 1.6 × 10−5
E)HCN + H2O ⇋ CN− + H3O+ Ka = 6.2 × 10−10
A)AgCN ⇋ Ag+ + CN−____________________Ksp = 2.2 × 10−16
B)H2O + H2O ⇋ H3O+ + OH− Kw = 1 × 10−14
C)[Ag+] = [CN−] + [HCN]
D)CN− + H2O ⇋ HCN + OH− Kb = 1.6 × 10−5
E)HCN + H2O ⇋ CN− + H3O+ Ka = 6.2 × 10−10

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
What is the ionic strength of a 2:1 electrolyte with a concentration of 0.100 M?
A)0)100
B)0)200
C)0)300
D)0)400
E)0)500
A)0)100
B)0)200
C)0)300
D)0)400
E)0)500

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is NOT true for pH?
A)pH = −log[H+]H+.
B)pH = −log[H+] when = 0.
C)The pH = pOH for pure water.
D)As ionic strength increases,so does the pH of pure water.
E)The ionic strength of pure water is 1.0 x 10−7 M.
A)pH = −log[H+]H+.
B)pH = −log[H+] when = 0.
C)The pH = pOH for pure water.
D)As ionic strength increases,so does the pH of pure water.
E)The ionic strength of pure water is 1.0 x 10−7 M.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the activity coefficient for Fe2+ for an ionic strength of 0.034 M.For = 0.01 M, = 0.675 and for = 0.05 M, = 0.485.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
____________________ is a measure of the total concentration of ions in solution.
A)Ionic radius
B)Ionic density
C)Ionic degree
D)Ionic strength
E)Ionic atmosphere
A)Ionic radius
B)Ionic density
C)Ionic degree
D)Ionic strength
E)Ionic atmosphere

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the pOH of a 0.010 M HCl solution with an ionic strength of 0.10 M.H+ = 0.83,OH− = 0.76.
A)11.92
B)11.80
C)11.88
D)12.00
E)12.20
A)11.92
B)11.80
C)11.88
D)12.00
E)12.20

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement below is INCORRECT for the increased solubility of a sparingly soluble salt,AgCl,in an inert salt solution,NaNO3?
A)The ionic atmosphere around the silver cations becomes increasingly negative as the nitrate anions are attracted to the silver cations.
B)The ionic strength around the chloride anions becomes increasingly positive as the sodium cations are attracted to the chloride anions.
C)The ionic atmosphere increases the attraction between silver cations and chloride anions,increasing solubility by creating more AgCl (aq)in solution.
D)Ions continually diffuse into and out of the ionic atmosphere.
E)The negative ionic atmosphere around silver cations results in an overall positive charge less than the positive charge of the silver cations.
A)The ionic atmosphere around the silver cations becomes increasingly negative as the nitrate anions are attracted to the silver cations.
B)The ionic strength around the chloride anions becomes increasingly positive as the sodium cations are attracted to the chloride anions.
C)The ionic atmosphere increases the attraction between silver cations and chloride anions,increasing solubility by creating more AgCl (aq)in solution.
D)Ions continually diffuse into and out of the ionic atmosphere.
E)The negative ionic atmosphere around silver cations results in an overall positive charge less than the positive charge of the silver cations.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
To correct for the effect of ionic strength on chemical reactions,concentrations are replaced by____________________ in equilibrium expressions.
A)activity coefficients
B)ion activities
C)molecular activities
D)activities
E)active strength
A)activity coefficients
B)ion activities
C)molecular activities
D)activities
E)active strength

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the pH of a 0.001 M HCN solution.Ka = 6.2 × 10−10

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the pH of a 0.010 M CH3CO2H solution.Ka = 1.8 × 10−5 for acetic acid.
A)9)26
B)3)39
C)2)02
D)10.61
E)4)74
A)9)26
B)3)39
C)2)02
D)10.61
E)4)74

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement below is NOT correct for the pH of a 0.01 M NaCl solution versus the pH of a 0.01 M in FeSO4 solution?
A)The activity coefficient for H+ will be larger in 0.01 M NaCl than in 0.01 M FeSO4.
B)The activity coefficient for OH− will be larger in 0.01 M NaCl than in 0.01 M FeSO4.
C)The hydronium concentration in 0.01 M NaCl will equal the hydronium concentration in 0.01 M FeSO4.
D)The H+ activity in 0.01 M NaCl will not equal the H+ activity in 0.01 M FeSO4.
E)The OH− activity in 0.01 M NaCl will not equal the OH− activity in 0.01 M FeSO4.
A)The activity coefficient for H+ will be larger in 0.01 M NaCl than in 0.01 M FeSO4.
B)The activity coefficient for OH− will be larger in 0.01 M NaCl than in 0.01 M FeSO4.
C)The hydronium concentration in 0.01 M NaCl will equal the hydronium concentration in 0.01 M FeSO4.
D)The H+ activity in 0.01 M NaCl will not equal the H+ activity in 0.01 M FeSO4.
E)The OH− activity in 0.01 M NaCl will not equal the OH− activity in 0.01 M FeSO4.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
All of the following are TRUE for activities and activity coefficients,except:
A)activity for a chemical species is the product of concentration and activity coefficient.
B)the activity coefficient corrects for non-ideal behavior due to ionic strength.
C)as ionic strength increases,the value of the activity coefficient increases.
D)for ions,the activity coefficient approaches unity as the ionic strength approaches 0.
E)the activity coefficient for neutral molecules is approximately unity when the ionic strength is less than 0.1 M.
A)activity for a chemical species is the product of concentration and activity coefficient.
B)the activity coefficient corrects for non-ideal behavior due to ionic strength.
C)as ionic strength increases,the value of the activity coefficient increases.
D)for ions,the activity coefficient approaches unity as the ionic strength approaches 0.
E)the activity coefficient for neutral molecules is approximately unity when the ionic strength is less than 0.1 M.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the statements below are INCORRECT for mass balance and charge balance?
I The mass balance is a statement of the conservation of matter.
II Mass balance is satisfied when the quantity of all species in a solution containing a particular atom (or group of atoms)equals the amount of that atom (or group)reacted.
III The sum of the positive charges in solution equals the sum of the negative charges in solution.
IV The coefficient in front of each species in a charge balance always equals the magnitude of the charge on the counter ion.
A)I,II,III and IV
B)I,III and IV
C)I,II and III
D)I and IV
E)II and III
I The mass balance is a statement of the conservation of matter.
II Mass balance is satisfied when the quantity of all species in a solution containing a particular atom (or group of atoms)equals the amount of that atom (or group)reacted.
III The sum of the positive charges in solution equals the sum of the negative charges in solution.
IV The coefficient in front of each species in a charge balance always equals the magnitude of the charge on the counter ion.
A)I,II,III and IV
B)I,III and IV
C)I,II and III
D)I and IV
E)II and III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


