Deck 11: Polyprotic Acid-Base Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 11: Polyprotic Acid-Base Equilibria
1
Calculate the pH of a 0.200 M H2A− solution.K1 = 1 × 10−4,K2 = 1 × 10−8 and K3 = 1 × 10−12.
A)4)35
B)6)00
C)8)00
D)8)65
E)10.00
A)4)35
B)6)00
C)8)00
D)8)65
E)10.00
10.00
2
Solid NaOH is added to 1 L of an isoleucine solution,H2L+,i.e,100 mM.When 175 mmol of NaOH is added,the principal species is____________________ .The pKa for the carboxylic acid group is 2.318 and the pKa for the ammonium group is 9.758.Assume the isoleucine solution volume does not change appreciably.
A)H2L+
B)L−
C)HL
D)H3L2+
E)L2−
A)H2L+
B)L−
C)HL
D)H3L2+
E)L2−
L−
3
Calculate the concentration of each species of oxalic acid in a 0.150 M solution of sodium oxalate,Na2C2O4.K1 = 5.62 × 10−2,K2 = 7.24 × 10−5
[A2−] = 0.150 M,[HA−] = 4.55 × 10−6 M,[H2A] = 1.78 × 10−13
4
Calculate the pH for a 0.200 M sodium L−aspartic acid solution in the H2L form.pKa1 = 1.990,pKa2 = 3.900 and pKa3 = 10.002.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Malonic acid is a diprotic acid with pKs of 2.847 and 5.696.Calculate the for each malonic acid species in solution at pH =3.50.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
The fractional composition diagram below is representative for diprotic weak acids.Which point INCORRECTLY indicates the principal species?
A)H2A
B)HA−
C)A−
D)H2A = HA−
E)HA− = A2−
A)H2A
B)HA−
C)A−
D)H2A = HA−
E)HA− = A2−

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Alanine is a diprotic amino acid with a pKa = 2.344 for the carboxylic group and a pKa = 9.868 for the ammonium group.At pH = 6.32,what is the principal species in solution?
A)H2L+
B)L−
C)HL
D)H3L2+
E)L2−
A)H2L+
B)L−
C)HL
D)H3L2+
E)L2−

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the answers below is INCORRECT for diprotic acids and bases?
A)Solutions of H2A are treated as monoprotic to calculate [H+],[HA−] and [H2A].
B)The pH for HA− solutions is appro×imated with pH = ½(pK1 + pK2).
C)Ka1Kb1 = Kw and Ka2Kb2 = Kw
D)Solutions of A2− is treated as monobasic to calculate [OH−],[HA−],and [A2−].
E)A zwitterion is a neutral molecule with both positive and negative charges.
A)Solutions of H2A are treated as monoprotic to calculate [H+],[HA−] and [H2A].
B)The pH for HA− solutions is appro×imated with pH = ½(pK1 + pK2).
C)Ka1Kb1 = Kw and Ka2Kb2 = Kw
D)Solutions of A2− is treated as monobasic to calculate [OH−],[HA−],and [A2−].
E)A zwitterion is a neutral molecule with both positive and negative charges.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the pH of a solution prepared by mixing 200.0 mL of 0.100 M NaOH with 150.0 mL of 0.200 M o×alic acid.K1 = 5.62 × 10−2,K2 = 7.24 × 10−5

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is TRUE for an aqueous solution of the weak acid H3A?
I H3A is acidic
II H2A− is acidic
III HA2− is amphiprotic
IV A3− is basic
A)II,III,and IV
B)I,II,and IV
C)I,II,and III
D)I,III,and IV
E)I,II,III,and IV
I H3A is acidic
II H2A− is acidic
III HA2− is amphiprotic
IV A3− is basic
A)II,III,and IV
B)I,II,and IV
C)I,II,and III
D)I,III,and IV
E)I,II,III,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the pH of a 0.340 M NaO2CCO2H solution.pK1 = 1.250 and pK2 = 4.266.
A)2)37
B)4)89
C)2)79
D)3)38
E)−0.029
A)2)37
B)4)89
C)2)79
D)3)38
E)−0.029

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
For the polyprotic acid H3A,which of the equilibria below is INCORRECT?
A)A3− ⇋ HA2− + OH− Kb3 = Kw/Ka3
B)H2A− ⇋ HA2− + H+ Ka2 = K2
C)HA2− ⇋ A3− + H+ Ka3 = K3
D)H3A ⇋ H2A− + H+ Ka1 = K1
E)HA2− ⇋ H2A− + OH− Kb2 = Kw/Ka2
A)A3− ⇋ HA2− + OH− Kb3 = Kw/Ka3
B)H2A− ⇋ HA2− + H+ Ka2 = K2
C)HA2− ⇋ A3− + H+ Ka3 = K3
D)H3A ⇋ H2A− + H+ Ka1 = K1
E)HA2− ⇋ H2A− + OH− Kb2 = Kw/Ka2

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Sulfurous acid is a diprotic acid with Ka1 = 1.40 × 10−2 and Ka2 = 6.73 × 10−8.Which of the statements below are FALSE?
I Kb2 = 7.14 × 10−13
II Kb1 = 1.49 × 10−7
III For 0.1 M H2SO3,[H+] = 7.61 × 10−2 for Ka1.
IV For 0.1 M NaHSO3,pH 4.515.
A)I,II,and IV
B)I,II,and III
C)I and II
D)I,II,III,and IV
E)II,III,and IV
I Kb2 = 7.14 × 10−13
II Kb1 = 1.49 × 10−7
III For 0.1 M H2SO3,[H+] = 7.61 × 10−2 for Ka1.
IV For 0.1 M NaHSO3,pH 4.515.
A)I,II,and IV
B)I,II,and III
C)I and II
D)I,II,III,and IV
E)II,III,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Sodium hydroxide is added to a 0.1 M solution of phenylalanine.Phenylalanine is a diprotic acid with two pKas,2.20 for the carboxylic acid group and 9.31 for the ammonium functional group.When the pH = 5.93,the principal species is____________________ and when the pH = 10.10,the principal species is____________________ .
A)H2L+;H2L+
B)HL;L−
C)H2L+;L−
D)H2L+;HL
E)HL;HL
A)H2L+;H2L+
B)HL;L−
C)H2L+;L−
D)H2L+;HL
E)HL;HL

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the pH at the isoelectric point for a 0.10 M solution of the amino acid threonine.pKa1 = 2.088 and pKa2 = 9.100 for threonine.
A)3)57
B)2)39
C)5)59
D)1)54
E)5)05
A)3)57
B)2)39
C)5)59
D)1)54
E)5)05

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Which is NOT true for isoelectric and isoionic pH?
A)Isoionic pH is the pH of the pure,neutral,polyprotic acid.
B)Isoelectric pH is the pH at which average charge of the polyprotic acid is +1.
C)The hydronium concentration at the isoionic point is calculated with the equation
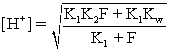
)
D)The pH at the isoelectric point is calculated with the equation pH = ½(pK1 + pK2).
E)For the equation
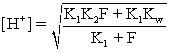
,F is the formal concentration of the weak acid.
A)Isoionic pH is the pH of the pure,neutral,polyprotic acid.
B)Isoelectric pH is the pH at which average charge of the polyprotic acid is +1.
C)The hydronium concentration at the isoionic point is calculated with the equation
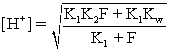
)
D)The pH at the isoelectric point is calculated with the equation pH = ½(pK1 + pK2).
E)For the equation
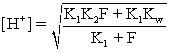
,F is the formal concentration of the weak acid.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
A buffer is prepared by dissolving 70 millimoles of solid NaOH in 500.0 mL of solution with a concentration of 50 mM H2A.Which of the following are NOT true for the buffer?
I After reaction there is 30 mmol HA− and 20 mmol A2− in solution.
II pH is calculated using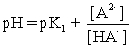 .
.
III After reaction there is 50 mmol HA− and 20 mmol NaOH in solution.
IV pH is calculated using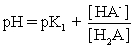 .
.
V pH = 14 - pOH where pOH is calculated from excess OH−.
A)III,IV,and V
B)II,III,IV,and V
C)1,III,and IV
D)I,II,and V
E)I and II
I After reaction there is 30 mmol HA− and 20 mmol A2− in solution.
II pH is calculated using
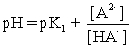 .
.III After reaction there is 50 mmol HA− and 20 mmol NaOH in solution.
IV pH is calculated using
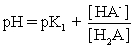 .
.V pH = 14 - pOH where pOH is calculated from excess OH−.
A)III,IV,and V
B)II,III,IV,and V
C)1,III,and IV
D)I,II,and V
E)I and II

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
For a weak acid,the HA____________________ and the A-____________________ with increased pH.
A)increases;decreases
B)increases;increases
C)decreases;decreases
D)decreases;increases
E)is fixed ;is fixed
A)increases;decreases
B)increases;increases
C)decreases;decreases
D)decreases;increases
E)is fixed ;is fixed

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the pH to which a malonic acid solution must be adjusted to reach the isoelectric point.pK1 = 2.847 and pK2 = 5.696.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
How many millimoles of NaOH or HCl must be added to 500.0 mL of 0.250 M NaO2CCO2H to prepare a pH 3.90 buffer? Assume no change in volume.pK1 = 1.250 and pK2 = 4.266.
A)37.65 mmol NaOH
B)87.35 mmol HCl
C)124.7 mmol HCl
D)0)30 mmol NaOH
E)The answer cannot be calculated from given information.
A)37.65 mmol NaOH
B)87.35 mmol HCl
C)124.7 mmol HCl
D)0)30 mmol NaOH
E)The answer cannot be calculated from given information.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


