Deck 16: Electrodes and Potentiometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 16: Electrodes and Potentiometry
1
Which is NOT a class of ion-selective electrode?
A)glass membranes
B)solid-state electrodes
C)liquid-based electrodes
D)compound electrodes
E)biochemical electrodes
A)glass membranes
B)solid-state electrodes
C)liquid-based electrodes
D)compound electrodes
E)biochemical electrodes
biochemical electrodes
2
An electrode has a potential of 1.201 V with respect to a saturated silver-silver chloride electrode.What would the electrodes potential be relative to a saturated calomel electrode (S.C.E. )? E = 0.197 V for the saturated silver-silver chloride electrode and E = 0.241 V for the S.C.E.
A)1)442
B)1)245 V
C)1)004 V
D)1)398 V
E)1)157 V
A)1)442
B)1)245 V
C)1)004 V
D)1)398 V
E)1)157 V
1)157 V
3
The potential of a silver electrode is measured relative to an Ag-AgCl electrode for the titration of 100.0 mL of 0.100 M Cl with 0.100 M Ag+.What is the potential after 75.00 mL of titrant is added.Eo = 0.799 V for Ag+,E = 0.197 V for the Ag-AgCl electrode and Ksp = 1.8 × 10−10.
A)0)493 V
B)1)070 V
C)0)521 V
D)1)267 V
E)0)134 V
A)0)493 V
B)1)070 V
C)0)521 V
D)1)267 V
E)0)134 V
0)134 V
4
The selectivity coefficient for a fluoride ion-selective electrode is 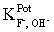 = 0.15.What will the change in the electrode potential be for a 1.4 × 10−5 M F− at pH 5.5 if the pH is raised to pH 11.0?
= 0.15.What will the change in the electrode potential be for a 1.4 × 10−5 M F− at pH 5.5 if the pH is raised to pH 11.0?
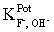 = 0.15.What will the change in the electrode potential be for a 1.4 × 10−5 M F− at pH 5.5 if the pH is raised to pH 11.0?
= 0.15.What will the change in the electrode potential be for a 1.4 × 10−5 M F− at pH 5.5 if the pH is raised to pH 11.0?
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
To compensate for a complex or unknown matrix,the____________________ method can be used to determine the analyte concentration.
A)calibration curve
B)standard addition
C)standardization
D)least-squares analysis
E)dilution method
A)calibration curve
B)standard addition
C)standardization
D)least-squares analysis
E)dilution method

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
A sodium selective electrode increased in voltage by 59.16 mV when the sodium cation concentration was increased by a factor of 10.A S2− selective electrode measured a decrease of 29.58 mL when the sulfide concentration increased by a factor of 10.Why?
I Cations always increase the measured potential by n × 59.16 mV where n is the charge on the cation.
II Anions always decrease the measured potential by n × 59.16 mV where n is the charge on the anion.
III Cations always increase the measured potential by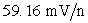 where n is the charge on the cation.
where n is the charge on the cation.
IV Anions always decrease the measured potential by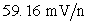 where n is the charge on the anion.
where n is the charge on the anion.
A)II and III
B)III and IV
C)I and II
D)I and IV
E)None of the statements explain the behavior.
I Cations always increase the measured potential by n × 59.16 mV where n is the charge on the cation.
II Anions always decrease the measured potential by n × 59.16 mV where n is the charge on the anion.
III Cations always increase the measured potential by
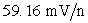 where n is the charge on the cation.
where n is the charge on the cation.IV Anions always decrease the measured potential by
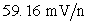 where n is the charge on the anion.
where n is the charge on the anion.A)II and III
B)III and IV
C)I and II
D)I and IV
E)None of the statements explain the behavior.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate E for the titration of 50.00 mL of 0.100 M Ag+ when 50.00 mL of 0.150 M Cl− is added.Silver wire is the indicator electrode and the saturated Ag-AgCl electrode is the reference electrode.E = 0.197 V for the saturated Ag-AgCl electrode, 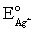 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
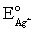 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
A 0.100 M KCl solution is placed in contact with a 0.100 M LiCl solution.Which of the following is true for junction between the two solutions?
I The KCl side of the junction will be positive as Cl− diffuses faster across the junction than K+.
II The LiCl side of the junction will be negative as Li+ diffuses faster across the junction than K+.
III The KCl side of the junction will be positive as K+ diffuses slower across the junction than Li+
IV The LiCl side of the junction will be more negative as Li+ diffuses faster across the junction than Cl−.
A)I and III
B)II and IV
C)I and II
D)III and IV
E)II and III
I The KCl side of the junction will be positive as Cl− diffuses faster across the junction than K+.
II The LiCl side of the junction will be negative as Li+ diffuses faster across the junction than K+.
III The KCl side of the junction will be positive as K+ diffuses slower across the junction than Li+
IV The LiCl side of the junction will be more negative as Li+ diffuses faster across the junction than Cl−.
A)I and III
B)II and IV
C)I and II
D)III and IV
E)II and III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
For indicator electrodes,which statements are true?
I Indicator electrodes are made from relatively inert metals.
II Indicator electrodes work best when the electrode surface is large and clean.
III Platinum is a common indicator electrode.
IV Platinum electrodes are more inert than gold electrodes.
A)I,III,and IV
B)II and III
C)I,II,and IV
D)I,II,and III
E)I,II,III,and IV
I Indicator electrodes are made from relatively inert metals.
II Indicator electrodes work best when the electrode surface is large and clean.
III Platinum is a common indicator electrode.
IV Platinum electrodes are more inert than gold electrodes.
A)I,III,and IV
B)II and III
C)I,II,and IV
D)I,II,and III
E)I,II,III,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which is NOT an error associated with pH measurement that is incorrectly defined?
A)junction potential drift - AgCl or Ag precipitates from solution due to dilution of the KCl or the presence of reducing agents
B)sodium error - electrode responds to Na+ instead of H+ when [Na+] is low and [H+] is high
C)acid error - the measured pH is higher than the actual pH because the glass is saturated with H+ and cannot be further protonated
D)temperature - pH meter calibrated at a different temperature than the sample
E)equilibration time - the electrode did not have sufficient time to equilibrate with the solution
A)junction potential drift - AgCl or Ag precipitates from solution due to dilution of the KCl or the presence of reducing agents
B)sodium error - electrode responds to Na+ instead of H+ when [Na+] is low and [H+] is high
C)acid error - the measured pH is higher than the actual pH because the glass is saturated with H+ and cannot be further protonated
D)temperature - pH meter calibrated at a different temperature than the sample
E)equilibration time - the electrode did not have sufficient time to equilibrate with the solution

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
The____________________ gives the relative response of an ion-selective electrode to different species with the same charge.
A)counter ion coefficient
B)isoionic coefficient
C)ion coefficient
D)selectivity coefficient
E)transient coefficient
A)counter ion coefficient
B)isoionic coefficient
C)ion coefficient
D)selectivity coefficient
E)transient coefficient

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
An electrode with a fixed potential is the:
A)cathode.
B)anode.
C)indicator electrode.
D)reference electrode.
E)working electrode.
A)cathode.
B)anode.
C)indicator electrode.
D)reference electrode.
E)working electrode.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Solid-state electrodes that respond to anions create a potential difference across the solid-state membrane by____________________ ,which allows anions to migrate through the crystal.
A)doping the membrane crystal to create anion vacancies
B)drilling small channels through the crystal
C)reacting the membrane crystal with a strong acid
D)polishing the surface of the membrane crystal
E)etching the surface of the membrane crystal with small grooves
A)doping the membrane crystal to create anion vacancies
B)drilling small channels through the crystal
C)reacting the membrane crystal with a strong acid
D)polishing the surface of the membrane crystal
E)etching the surface of the membrane crystal with small grooves

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Calculate E for the titration of 50.00 mL of 0.100 M Ag+ when 25.00 mL of 0.150 M Cl− is added.Silver wire is the indicator electrode and the saturated Ag-AgCl electrode is the reference electrode.E = 0.197 V for the saturated Ag-AgCl electrode, 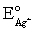 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
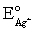 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
The____________________ is the voltage difference when two dissimilar electrolyte solutions are in contact.
A)ion potential
B)electrolyte potential
C)cation potential
D)junction potential
E)anion potential
A)ion potential
B)electrolyte potential
C)cation potential
D)junction potential
E)anion potential

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
A pH probe is calibrated against pH 4 and pH 7 buffers.For pH 4 buffer,E = 200 mV and for pH 7 buffer,E = 23 mV.If the potential for an unknown solution is 157 mV,calculate the pH of the solution.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
All of the following are true for ion-selective electrodes specific to aqueous ions,except:
I two reference electrodes measure the voltage difference across the ion-selective electrodes organic polymer membrane.
II the organic polymer membrane separates the inner solution from the outer analyte solution.
III the organic polymer membrane is hydrophobic and is impregnated with a viscous organic solution containing an ion exchanger and sometimes a ligand specific for the analyte ion.
IV the potential difference across the organic polymer membrane occurs when the analyte ions embedded in the membrane diffuse into the inner aqueous solution creating a junction potential.
V the potential difference is dependent only on the activity of analyte ion in the outer solution.
A)IV
B)I and V
C)I and IV
D)IV and V
E)V
I two reference electrodes measure the voltage difference across the ion-selective electrodes organic polymer membrane.
II the organic polymer membrane separates the inner solution from the outer analyte solution.
III the organic polymer membrane is hydrophobic and is impregnated with a viscous organic solution containing an ion exchanger and sometimes a ligand specific for the analyte ion.
IV the potential difference across the organic polymer membrane occurs when the analyte ions embedded in the membrane diffuse into the inner aqueous solution creating a junction potential.
V the potential difference is dependent only on the activity of analyte ion in the outer solution.
A)IV
B)I and V
C)I and IV
D)IV and V
E)V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
If the measured potential of a half-cell is 0.584 V when measured against the S.C.E,what is the potential if the measurement is made using the saturated Ag-AgCl reference electrode? E = 0.197 V for the saturated Ag-AgCl electrode and E = 0.241 V for the S.C.E.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Solid-state chemical sensors are manufactured using semiconductors such as Si and Ge.Doping enhances the conductivity of Si.Which is NOT true for doping of silicon?
A)Doping replaces some of the silicon atoms with atoms of different elements.
B)Doping silicon with phosphorous enhances silicon's conductivity by providing an extra conduction electron that is free to move through the crystal.
C)Doping silicon with aluminum enhances silicon's conductivity by creating a vacancy in the crystal called a hole,which behaves as a negative charge carrier.
D)n-type silicon has excess conduction electrons.
E)p-type silicon has excess holes.
A)Doping replaces some of the silicon atoms with atoms of different elements.
B)Doping silicon with phosphorous enhances silicon's conductivity by providing an extra conduction electron that is free to move through the crystal.
C)Doping silicon with aluminum enhances silicon's conductivity by creating a vacancy in the crystal called a hole,which behaves as a negative charge carrier.
D)n-type silicon has excess conduction electrons.
E)p-type silicon has excess holes.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
The main advantage to using a saturated KCl solution in reference electrodes is:
A)the low cost of potassium chloride.
B)the ease of reaction with analyte.
C)the chloride concentration does not change as liquid evaporates from the cell.
D)the ease of cell construction.
E)the ease at which chloride passes through membranes.
A)the low cost of potassium chloride.
B)the ease of reaction with analyte.
C)the chloride concentration does not change as liquid evaporates from the cell.
D)the ease of cell construction.
E)the ease at which chloride passes through membranes.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


