Deck 17: Redox Titrations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 17: Redox Titrations
1
The percent purity of a malonic acid reagent must be calculated.A student weighs out 0.1511 grams of malonic acid,CH2(CO2H)2,and quantitatively transfers the solid to a 250 mL Erlenmeyer flask.To the flask 50.00 mL of distilled water is added to dissolve the malonic acid.After dissolution,the student uses a 50 mL volumetric pipet to add 50 mL of 0.200 M Ce4+,which is allowed to react with the malonic acid.After complete reaction,the excess Ce4+ is back titrated with 14.58 mL of 0.100 M Fe2+ solution.Calculate the percent purity malonic acid.
CH2(CO2H)2 + 2 H2O + 6 Ce4+ → 2 CO2 + HCO2H + 6 Ce3+ + 6 H+
Ce4+ + Fe2+ ⇋ Ce3+ + Fe3+
CH2(CO2H)2 + 2 H2O + 6 Ce4+ → 2 CO2 + HCO2H + 6 Ce3+ + 6 H+
Ce4+ + Fe2+ ⇋ Ce3+ + Fe3+
98.0% pure malonic acid
2
Potassium permanganate is a strong oxidant commonly used in redox titrations.Which is(are)TRUE for permanganates titration chemistry?
I In strongly acidic solution the permanganate half-reaction is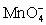 + 8 H+ + 5 e- ⇋ Mn2+ + 4 H2O.
+ 8 H+ + 5 e- ⇋ Mn2+ + 4 H2O.
II In neutral or alkaline solution the permanganate half-reaction is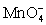 + 4 H+ + 3 e- ⇋ MnO2 (s)+ 2 H2O.
+ 4 H+ + 3 e- ⇋ MnO2 (s)+ 2 H2O.
III In strongly alkaline solution the permanganate half-reaction is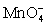 + e- ⇋
+ e- ⇋ 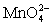 .
.
A)III only
B)II and III
C)I and III
D)I and II
E)I,II,and III
I In strongly acidic solution the permanganate half-reaction is
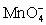 + 8 H+ + 5 e- ⇋ Mn2+ + 4 H2O.
+ 8 H+ + 5 e- ⇋ Mn2+ + 4 H2O.II In neutral or alkaline solution the permanganate half-reaction is
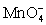 + 4 H+ + 3 e- ⇋ MnO2 (s)+ 2 H2O.
+ 4 H+ + 3 e- ⇋ MnO2 (s)+ 2 H2O.III In strongly alkaline solution the permanganate half-reaction is
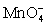 + e- ⇋
+ e- ⇋ 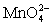 .
.A)III only
B)II and III
C)I and III
D)I and II
E)I,II,and III
I,II,and III
3
Sodium thiosulfate is used almost exclusively to titrate 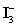 .Which of the following is NOT true for sodium thiosulfate titrant?
.Which of the following is NOT true for sodium thiosulfate titrant?
A)In neutral or acidic solution,triiodide oxidizes thiosulfate to tetrathionate.
B)One mole of
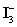
Is equivalent to two moles of I2.
C)In basic solution,
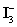
Disproportionates to I− and HOI,which can oxidize
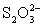
To
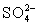
)
D)Sodium thiosulfate is standardized with freshly made
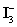
Solution prepared from KIO3 and KI.
E)Dissolved CO2 and metal ion catalyzed atmospheric oxidation of thiosulfate decreases the concentration of prepared thiosulfate solution.
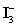 .Which of the following is NOT true for sodium thiosulfate titrant?
.Which of the following is NOT true for sodium thiosulfate titrant?A)In neutral or acidic solution,triiodide oxidizes thiosulfate to tetrathionate.
B)One mole of
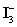
Is equivalent to two moles of I2.
C)In basic solution,
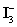
Disproportionates to I− and HOI,which can oxidize
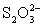
To
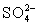
)
D)Sodium thiosulfate is standardized with freshly made
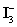
Solution prepared from KIO3 and KI.
E)Dissolved CO2 and metal ion catalyzed atmospheric oxidation of thiosulfate decreases the concentration of prepared thiosulfate solution.
One mole of
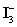
Is equivalent to two moles of I2.
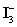
Is equivalent to two moles of I2.
4
Potassium permanganate is NOT a primary standard.Why?
I Solid KMnO4 contains traces of MnO2.
[![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_204e_a4b1_3707ba7a63a5_TB4000_11.jpg) actual] < [
actual] < [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_204f_a4b1_d9a31175adae_TB4000_11.jpg) calculated]
calculated]
II Permanganate reacts with organic material in distilled water.
[![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2050_a4b1_1b4e4f61d039_TB4000_11.jpg) actual] > [
actual] > [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2051_a4b1_815e14195bc4_TB4000_11.jpg) calculated]
calculated]
III Permanganate is unstable in aqueous solution,producing MnO2,O2,and OH−.
[![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2052_a4b1_79edd52c4e40_TB4000_11.jpg) actual] < [
actual] < [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2053_a4b1_3b88894d30c2_TB4000_11.jpg) calculated]
calculated]
IV The presence of MnO2,Mn2+,heat,light,acids,and bases increase the rate of reaction between permanganate and water.
[![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2054_a4b1_5db174fd1dcc_TB4000_11.jpg) actual] < [
actual] < [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_4665_a4b1_7124d00b3057_TB4000_11.jpg) calculated]
calculated]
A)I and II
B)I,II,and IV
C)II,III,and IV
D)I,III,and IV
E)I,II,and III
I Solid KMnO4 contains traces of MnO2.
[
![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_204e_a4b1_3707ba7a63a5_TB4000_11.jpg) actual] < [
actual] < [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_204f_a4b1_d9a31175adae_TB4000_11.jpg) calculated]
calculated]II Permanganate reacts with organic material in distilled water.
[
![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2050_a4b1_1b4e4f61d039_TB4000_11.jpg) actual] > [
actual] > [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2051_a4b1_815e14195bc4_TB4000_11.jpg) calculated]
calculated]III Permanganate is unstable in aqueous solution,producing MnO2,O2,and OH−.
[
![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2052_a4b1_79edd52c4e40_TB4000_11.jpg) actual] < [
actual] < [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2053_a4b1_3b88894d30c2_TB4000_11.jpg) calculated]
calculated]IV The presence of MnO2,Mn2+,heat,light,acids,and bases increase the rate of reaction between permanganate and water.
[
![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_2054_a4b1_5db174fd1dcc_TB4000_11.jpg) actual] < [
actual] < [ ![<strong>Potassium permanganate is NOT a primary standard.Why? I Solid KMnO<sub>4</sub> contains traces of MnO<sub>2</sub>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] II Permanganate reacts with organic material in distilled water. [ <sub>actual</sub>] > [ <sub>calculated</sub>] III Permanganate is unstable in aqueous solution,producing MnO<sub>2</sub>,O<sub>2</sub>,and OH<sup>−</sup>. [ <sub>actual</sub>] < [ <sub>calculated</sub>] IV The presence of MnO<sub>2</sub>,Mn<sup>2+</sup>,heat,light,acids,and bases increase the rate of reaction between permanganate and water. [ <sub>actual</sub>] < [ <sub>calculated</sub>]</strong> A)I and II B)I,II,and IV C)II,III,and IV D)I,III,and IV E)I,II,and III](https://storage.examlex.com/TB4000/11ea6952_25af_4665_a4b1_7124d00b3057_TB4000_11.jpg) calculated]
calculated]A)I and II
B)I,II,and IV
C)II,III,and IV
D)I,III,and IV
E)I,II,and III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
For redox titrations,the oxidation state of the analyte may need adjusting.The analyte can be preoxidized with an oxidizing agent to quantitatively oxidize the analyte,with the excess oxidizing agent eliminated to prevent interference with titration.Which of the oxidizing agents is paired with INCORRECT elimination chemistry?
A)Excess peroxydisulfate is eliminated by boiling,converting the peroxydisulfate to sulfate.
B)Excess silver(I,III)oxide in mineral acid is eliminated by boiling,converting silver(III)cation to silver(I)cation.
C)Excess solid sodium bismuthate is removed by filtration.
D)Excess hydrogen peroxide in basic solution is eliminated by boiling,converting hydrogen peroxide to water and oxygen gas.
E)Excess stannous chloride in hot HCl is eliminated by boiling,converting stannous cation to stannic cation.
A)Excess peroxydisulfate is eliminated by boiling,converting the peroxydisulfate to sulfate.
B)Excess silver(I,III)oxide in mineral acid is eliminated by boiling,converting silver(III)cation to silver(I)cation.
C)Excess solid sodium bismuthate is removed by filtration.
D)Excess hydrogen peroxide in basic solution is eliminated by boiling,converting hydrogen peroxide to water and oxygen gas.
E)Excess stannous chloride in hot HCl is eliminated by boiling,converting stannous cation to stannic cation.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
____________________ is the titration of iodine produced by a chemical reaction.
A)Iodigravimetric titration
B)Iodimetry
C)Iodogravimetric titration
D)Iodometry
E)Iodotration
A)Iodigravimetric titration
B)Iodimetry
C)Iodogravimetric titration
D)Iodometry
E)Iodotration

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the potential for a redox titration when 50.00 mL of 0.100 M Co3+ is titrated with 35.00 mL of 0.130 M 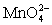 .The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V. Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V
.The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V. Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V 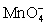 + e- ⇋
+ e- ⇋ 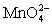 Eo = 0.56 V
Eo = 0.56 V
A)0)42 V
B)0)62 V
C)1)66 V
D)1)86 V
E)1)24 V
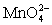 .The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V. Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V
.The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V. Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V 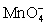 + e- ⇋
+ e- ⇋ 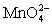 Eo = 0.56 V
Eo = 0.56 VA)0)42 V
B)0)62 V
C)1)66 V
D)1)86 V
E)1)24 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Starch is an indicator for iodine.For iodimetry,starch solution is added:
A)at the beginning of the titration.
B)at the half-way point of the titration.
C)right before the equivalence point of the titration.
D)after the equivalence point of the titration has been reached.
E)to the titrant before starting the titration.
A)at the beginning of the titration.
B)at the half-way point of the titration.
C)right before the equivalence point of the titration.
D)after the equivalence point of the titration has been reached.
E)to the titrant before starting the titration.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
A student is given the assignment to determine the mass iron in a brand of women's vitamins.He begins by taking four vitamin tablets,grinding the tablets into a fine powder to undergo the necessary sample preparation to free all iron from the sample matrix and to reduce all iron to the +2 state.The resulting Fe2+ solution is transferred to a 250 mL volumetric flask and diluted to volume with distilled water.A 50 mL aliquot of the sample requires 42.98 mL of 1.00 x 10−3 M 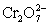 .Calculate the mg Fe per tablet.
.Calculate the mg Fe per tablet. 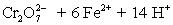 ⇋
⇋ 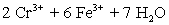
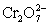 .Calculate the mg Fe per tablet.
.Calculate the mg Fe per tablet. 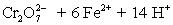 ⇋
⇋ 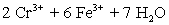

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the potential for a redox titration when 50.00 mL of 0.100 M Co3+ is titrated with 50.00 mL of 0.110 M 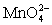 .The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V.
.The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V.
Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V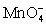 + e- ⇋
+ e- ⇋ 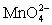 Eo = 0.56 V
Eo = 0.56 V
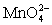 .The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V.
.The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V.Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V
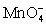 + e- ⇋
+ e- ⇋ 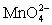 Eo = 0.56 V
Eo = 0.56 V
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the statements below are true for the titration of Fe2+ with standard Ce4+?
I The indicator electrode potential before the addition of Ce4+ solution is calculated using the Fe3+ half-reaction after calculating the equilibrium concentrations of Fe3+ and Fe2+ in solution.
II Before the equivalence point,the indicator electrode potential is calculated using the Fe3+ half-reaction because only [Fe3+] and [Fe2+] are known.
III At the equivalence point,the indicator potential is the average of Eo for Fe3+ and Ce4+.
IV After the equivalence point,the indicator electrode potential is calculated using the Ce4+ half-reaction because only [Ce4+] and [Ce2+] are known.
A)II and IV
B)II,III,and IV
C)I,III,and IV
D)I,II,and IV
E)I,II,III,and IV
I The indicator electrode potential before the addition of Ce4+ solution is calculated using the Fe3+ half-reaction after calculating the equilibrium concentrations of Fe3+ and Fe2+ in solution.
II Before the equivalence point,the indicator electrode potential is calculated using the Fe3+ half-reaction because only [Fe3+] and [Fe2+] are known.
III At the equivalence point,the indicator potential is the average of Eo for Fe3+ and Ce4+.
IV After the equivalence point,the indicator electrode potential is calculated using the Ce4+ half-reaction because only [Ce4+] and [Ce2+] are known.
A)II and IV
B)II,III,and IV
C)I,III,and IV
D)I,II,and IV
E)I,II,III,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
In addition to redox indicators and starch-iodine complex,the equivalence point for a redox titration can be determined using a Gran plot.Which is NOT true for Gran plots?
A)Gran plots give best results using redox data well before the equivalence point.
B)Gran plots are constructed by plotting the volume titrant added on the x-axis and V10−nE/0.05916 on the y-axis.
C)The plot of V versus V10−nE/0.05916 is a straight line with the x-intercept = equivalence point volume.
D)Constant ionic strength over the course of the titration minimizes error in the equivalence point.
E)Gran plots are more error prone than first derivative and second derivative plots.
A)Gran plots give best results using redox data well before the equivalence point.
B)Gran plots are constructed by plotting the volume titrant added on the x-axis and V10−nE/0.05916 on the y-axis.
C)The plot of V versus V10−nE/0.05916 is a straight line with the x-intercept = equivalence point volume.
D)Constant ionic strength over the course of the titration minimizes error in the equivalence point.
E)Gran plots are more error prone than first derivative and second derivative plots.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Which statements are true for redox indicators?
I.The color change occurs when the indicator goes from its oxidized form to its reduced form.
II. The color for the oxidized state of the indicator occurs when the ratio of the reduced state to the oxidized state is 1:10.
III. The range over which the indicator functions is Eo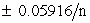 .
.
IV. The color for the reduced state of the indicator occurs when the ratio of the reduced state to the oxidized state is is 1:10.
A)I,II,III,and IV
B)III and IV
C)I,III,and IV
D)I,II,and IV
E)I,II,and III
I.The color change occurs when the indicator goes from its oxidized form to its reduced form.
II. The color for the oxidized state of the indicator occurs when the ratio of the reduced state to the oxidized state is 1:10.
III. The range over which the indicator functions is Eo
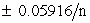 .
.IV. The color for the reduced state of the indicator occurs when the ratio of the reduced state to the oxidized state is is 1:10.
A)I,II,III,and IV
B)III and IV
C)I,III,and IV
D)I,II,and IV
E)I,II,and III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Which are TRUE for the use of indicators with dichromate?
I Dichromate is orange and changes color to green when reduced.The color change is abrupt enough to serve as an indicator.
II The equivalence point for dichromate can be determined using Pt and calomel electrodes.
III Dichromate end points are determined using the distinct color changes of diphenylamine sulfonic acid or diphenylbenzidine sulfonic acid indicators.
IV In basic solution,the orange dichromate solution changes color to yellow when reduced to chromate anion.The color change is abrupt enough to serve as an indicator.
A)II and III
B)I and IV
C)I,II,and IV
D)I and III
E)II and IV
I Dichromate is orange and changes color to green when reduced.The color change is abrupt enough to serve as an indicator.
II The equivalence point for dichromate can be determined using Pt and calomel electrodes.
III Dichromate end points are determined using the distinct color changes of diphenylamine sulfonic acid or diphenylbenzidine sulfonic acid indicators.
IV In basic solution,the orange dichromate solution changes color to yellow when reduced to chromate anion.The color change is abrupt enough to serve as an indicator.
A)II and III
B)I and IV
C)I,II,and IV
D)I and III
E)II and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
A 50.00 mL aliquot of a water sample is reacted with excess potassium iodide in acidic solution to generate 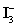 .Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the
.Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the 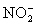 concentration in ppm? Assume solution density of 1.000 g/mL.
concentration in ppm? Assume solution density of 1.000 g/mL. 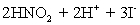 ⇋
⇋ 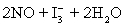
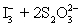 ⇋
⇋ 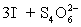
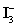 .Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the
.Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the 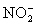 concentration in ppm? Assume solution density of 1.000 g/mL.
concentration in ppm? Assume solution density of 1.000 g/mL. 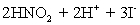 ⇋
⇋ 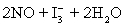
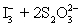 ⇋
⇋ 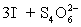

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Cerium(IV)is a strong oxidizing agent commonly used in redox titrations.Which of the following is NOT true for cerium(IV)?
A)Ce4+ is yellow and Ce3+ is colorless.The color change is abrupt enough that Ce4+ can serve as its own indicator.
B)Reduction of Ce4+ to Ce3+ proceeds cleanly in acidic solutions.
C)Ce4+ does not form aqueous complexes when dissolved in water.
D)Ce4+ is indefinitely stable in sulfuric acid solution.
E)Ce4+ reacts rapidly with chloride in hot hydrochloric acid solutions to form chlorine gas.
A)Ce4+ is yellow and Ce3+ is colorless.The color change is abrupt enough that Ce4+ can serve as its own indicator.
B)Reduction of Ce4+ to Ce3+ proceeds cleanly in acidic solutions.
C)Ce4+ does not form aqueous complexes when dissolved in water.
D)Ce4+ is indefinitely stable in sulfuric acid solution.
E)Ce4+ reacts rapidly with chloride in hot hydrochloric acid solutions to form chlorine gas.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
For redox titrations,the oxidation state of the analyte may need adjusting.The analyte can be prereduced with a reducing agent to quantitatively reduce the analyte,with the excess reducing agent eliminated to prevent interference with titration.Which of the reducing agents is paired with INCORRECT elimination chemistry?
A)Stannous chloride in hot HCl is eliminated by the addition of excess HgCl2,converting stannous cation to stannic cation.
B)Chromous chloride is eliminated by boiling,converting chromium(II)cation to chromium(III).
C)Sulfur dioxide is expelled by boiling in acid.
D)Hydrogen sulfide is expelled by boiling in base.
E)Excess hydrogen peroxide in acidic solution is eliminated by boiling,converting hydrogen peroxide to water and oxygen gas.
A)Stannous chloride in hot HCl is eliminated by the addition of excess HgCl2,converting stannous cation to stannic cation.
B)Chromous chloride is eliminated by boiling,converting chromium(II)cation to chromium(III).
C)Sulfur dioxide is expelled by boiling in acid.
D)Hydrogen sulfide is expelled by boiling in base.
E)Excess hydrogen peroxide in acidic solution is eliminated by boiling,converting hydrogen peroxide to water and oxygen gas.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
A Ce4+ standard solution is prepared with primary-standard-grade ammonium hexanitratocerate(IV),(NH4)2Ce(NO3)6.Which of the follow will NOT decrease the concentration of Ce4+ in solution over time?
A)Ce4+ solution prepared in distilled water.
B)Ce4+ prepared in hot HCl solution.
C)Ce4+ prepared in H2SO4 solution.
D)Ce4+ prepared in HClO4 solution.
E)Ce4+ prepared in HNO3 solution.
A)Ce4+ solution prepared in distilled water.
B)Ce4+ prepared in hot HCl solution.
C)Ce4+ prepared in H2SO4 solution.
D)Ce4+ prepared in HClO4 solution.
E)Ce4+ prepared in HNO3 solution.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
A permanganate solution is prepared by dissolving 20.0123 g KMnO4 in 500 mL of distilled water and boiled for 1 hour to remove any organic material.Following sintered-glass filtration,the solution is quantitatively transferred to a 1.0 L volumetric flask and diluted to volume with distilled water.The permanganate solution was titrated against 0.1023 M oxalic acid prepared in sulfuric acid solution.A 50.00 mL aliquot of oxalic acid solution required 16.68 mL of permanganate solution.A titration blank required 0.04 mL of permanganate.What is the permanganate molarity? 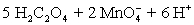 ⇋
⇋ 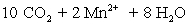
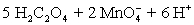 ⇋
⇋ 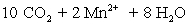

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Which is NOT true for oxidation with potassium dichromate?
A)In acid solution,dichromate ion is orange.
B)In acid solution,dichromate ion is reduced to chromous ion.
C)Dichromate is a less powerful oxidizing agent than permanganate and cerium(IV).
D)In basic solution,dichromate is converted into yellow chromate ion.
E)Potassium dichromate is a primary standard.
A)In acid solution,dichromate ion is orange.
B)In acid solution,dichromate ion is reduced to chromous ion.
C)Dichromate is a less powerful oxidizing agent than permanganate and cerium(IV).
D)In basic solution,dichromate is converted into yellow chromate ion.
E)Potassium dichromate is a primary standard.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


