Deck 18: Electroanalytical Techniques
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 18: Electroanalytical Techniques
1
Molecules have three ways to reach the surface of an electrode: diffusion,convection,and migration.The rotating disk electrode uses which of these three methods to control the flux of analyte that reaches the electrode?
A)convection
B)diffusion and convection
C)migration
D)convection and migration
E)diffusion and migration
A)convection
B)diffusion and convection
C)migration
D)convection and migration
E)diffusion and migration
diffusion and convection
2
The Karl Fischer titration measures the traces of water in samples such as transformer oils,foods,polymers,and so forth.Which of the following is NOT true for the chemistry involved?
I The anode solution contains iodide;an alcohol,ROH;a base,B;sulfur dioxide;and possibly other organic compounds.
II ROH + SO2 + B → BH+ +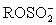 III I2 is generated from I− at the cathode.
III I2 is generated from I− at the cathode.
IV H2O + I2 +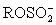 + 2 B →
+ 2 B → 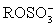 + 2 BH+I-
+ 2 BH+I-
V Two moles of e− corresponds to one mole H2O
A)III only
B)I and III
C)I,III,and V
D)II and IV
E)V only
I The anode solution contains iodide;an alcohol,ROH;a base,B;sulfur dioxide;and possibly other organic compounds.
II ROH + SO2 + B → BH+ +
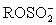 III I2 is generated from I− at the cathode.
III I2 is generated from I− at the cathode.IV H2O + I2 +
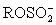 + 2 B →
+ 2 B → 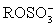 + 2 BH+I-
+ 2 BH+I-V Two moles of e− corresponds to one mole H2O
A)III only
B)I and III
C)I,III,and V
D)II and IV
E)V only
III only
3
Which is NOT true of electrogravimetric analysis?
A)Analyte is quantitatively deposited on an electrode via electrolysis.
B)The greater the analyte mass deposited correlates with the amount of analyte in solution.
C)If the analyte has a color in solution;disappearance of the color indicates completion of deposition.
D)If additional electrode surface is exposed to the solution and further deposition does not occur,the deposition of analyte is complete.
E)If a qualitative test for a small sample of the analyte solution tests positive,the deposition of analyte is complete.
A)Analyte is quantitatively deposited on an electrode via electrolysis.
B)The greater the analyte mass deposited correlates with the amount of analyte in solution.
C)If the analyte has a color in solution;disappearance of the color indicates completion of deposition.
D)If additional electrode surface is exposed to the solution and further deposition does not occur,the deposition of analyte is complete.
E)If a qualitative test for a small sample of the analyte solution tests positive,the deposition of analyte is complete.
If a qualitative test for a small sample of the analyte solution tests positive,the deposition of analyte is complete.
4
For a three-electrode cell the working electrode is the electrode at which the reaction of interest occurs.The____________________ is used to measure the potential of the working electrode and the____________________ is the current-supporting partner of the working electrode.
A)auxiliary electrode;reference electrode
B)reference electrode;auxiliary electrode
C)reference electrode;polarizable electrode
D)cathode;anode
E)potential electrode;reference electrode
A)auxiliary electrode;reference electrode
B)reference electrode;auxiliary electrode
C)reference electrode;polarizable electrode
D)cathode;anode
E)potential electrode;reference electrode

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
When a potential is applied to a solution that is 0.10 M Ni2+ and 0.20 M Fe2+,which metal will be reduced first and at what potential? Is it possible to remove the metal from solution with 99.99% efficiency before the second metal begins to be reduced from solution?
Ni2+ + 2 e- ⇋ Ni,Eo = -0.236 V
Fe2+ + 2 e- ⇋ Fe,Eo = -0.44 V
Ni2+ + 2 e- ⇋ Ni,Eo = -0.236 V
Fe2+ + 2 e- ⇋ Fe,Eo = -0.44 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
Which are true for polarography?
I Polarography is amperometry conducted with a dropping Hg electrode.
II The diffusion current in the plateau region of the polarographic wave is proportional to the concentration of the analyte and is used for quantitative analysis.
III The half-wave potential for maximum current is characteristic of a given analyte in a given medium and can be used for qualitative analysis for the analyte.
IV Fresh Hg drops give reproducible results.
V Polarography is used to primarily to study oxidations Hg may reduce when studying reductions.
A)I and V
B)II,III,and V
C)I,III,and IV
D)III,IV,and V
E)II,III,and IV
I Polarography is amperometry conducted with a dropping Hg electrode.
II The diffusion current in the plateau region of the polarographic wave is proportional to the concentration of the analyte and is used for quantitative analysis.
III The half-wave potential for maximum current is characteristic of a given analyte in a given medium and can be used for qualitative analysis for the analyte.
IV Fresh Hg drops give reproducible results.
V Polarography is used to primarily to study oxidations Hg may reduce when studying reductions.
A)I and V
B)II,III,and V
C)I,III,and IV
D)III,IV,and V
E)II,III,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
The____________________ is the electrode at which the reaction of interest occurs.
A)auxillary electrode
B)counter electrode
C)anode
D)cathode
E)working electrode
A)auxillary electrode
B)counter electrode
C)anode
D)cathode
E)working electrode

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement(s)is(are)defined INCORRECTLY?
I Ohmic potential is the voltage to overcome the electrical resistance of the solution in an electrochemical cell when a current is flowing.
II Concentrational polarization occurs when the concentrations of the reactants or products at the surface of an electrode is greater than those in the bulk solution.
III Overpotential is the voltage required to overcome the activation energy for a reaction at only the cathode.
IV E = E(cathode)- E(anode)- IR - overpotentials - concentration polarization
A)II,III,and IV
B)I,II,and III
C)Only III
D)II and IV
E)I and II
I Ohmic potential is the voltage to overcome the electrical resistance of the solution in an electrochemical cell when a current is flowing.
II Concentrational polarization occurs when the concentrations of the reactants or products at the surface of an electrode is greater than those in the bulk solution.
III Overpotential is the voltage required to overcome the activation energy for a reaction at only the cathode.
IV E = E(cathode)- E(anode)- IR - overpotentials - concentration polarization
A)II,III,and IV
B)I,II,and III
C)Only III
D)II and IV
E)I and II

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
An experiment measured the cathodic current for five solutions of known 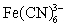 concentration using cyclic voltammetry.The collected data are plotted and the best line fit applied.If an unknown ferricyanide solution has a cathodic current of 17.38 A,calculate the concentration ferricyanide in the unknown.
concentration using cyclic voltammetry.The collected data are plotted and the best line fit applied.If an unknown ferricyanide solution has a cathodic current of 17.38 A,calculate the concentration ferricyanide in the unknown. 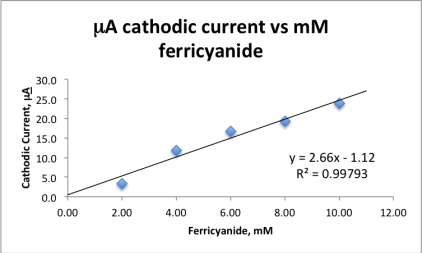
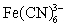 concentration using cyclic voltammetry.The collected data are plotted and the best line fit applied.If an unknown ferricyanide solution has a cathodic current of 17.38 A,calculate the concentration ferricyanide in the unknown.
concentration using cyclic voltammetry.The collected data are plotted and the best line fit applied.If an unknown ferricyanide solution has a cathodic current of 17.38 A,calculate the concentration ferricyanide in the unknown. 

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which is NOT true of overpotential?
A)Overpotential is the voltage required to overcome the activation energy for a reaction at an electrode.
B)The rate of the reaction will increase as the applied overpotential increases.
C)Activation energy,hence overpotential,is electrode-surface independent.
D)Applying an increasing overpotential will sustain a higher current density.
E)Current density is current per unit area of electrode surface,A/m2.
A)Overpotential is the voltage required to overcome the activation energy for a reaction at an electrode.
B)The rate of the reaction will increase as the applied overpotential increases.
C)Activation energy,hence overpotential,is electrode-surface independent.
D)Applying an increasing overpotential will sustain a higher current density.
E)Current density is current per unit area of electrode surface,A/m2.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
A technician is tasked with testing the newly purchased chloride autotitrator.He prepares a solution that is 3.4238 mg NaCl/mL solution,taking a 25.00 mL aliquot for titration.To minimize any problems with the in situ generation of silver cation titrant,a fresh silver anode is installed and the instrument is set to titrate with a constant current of 500.0 mA.The instrument took 286.3 seconds to reach the end point.Calculate the percent error for the instrument.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
____________________ is a set of techniques that observes the relationship between current and voltage during an electrochemical process.
A)Amperometry
B)Coulometry
C)Electrophoresis
D)Voltammetry
E)Electrogravimetric analysis
A)Amperometry
B)Coulometry
C)Electrophoresis
D)Voltammetry
E)Electrogravimetric analysis

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Which is NOT true for square wave voltammetry,stripping analysis,and cyclic voltammetry?
A)Square wave voltammetry applies a square waveform superimposed on a staircase waveform of increasing potential to the working electrode.
B)Stripping analysis is the most sensitive of the voltammetric techniques.
C)Cyclic voltammetry applies a triangular shaped waveform to the working electrode.
D)For cathodic stripping analysis,the peak current during oxidation is proportional to analyte concentration.
E)For cyclic voltammetry,for a reversible reaction the peak current,Ipc,for the forward sweep of the first cycle is proportional to the concentration of analyte and the square root of the sweep rate.
A)Square wave voltammetry applies a square waveform superimposed on a staircase waveform of increasing potential to the working electrode.
B)Stripping analysis is the most sensitive of the voltammetric techniques.
C)Cyclic voltammetry applies a triangular shaped waveform to the working electrode.
D)For cathodic stripping analysis,the peak current during oxidation is proportional to analyte concentration.
E)For cyclic voltammetry,for a reversible reaction the peak current,Ipc,for the forward sweep of the first cycle is proportional to the concentration of analyte and the square root of the sweep rate.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Sulfide in a water sample is titrated with electrolytically generated Fe2+.If a 10.00 mL aliquot of the water sample requires a current of 0.75 A for 5.4 minutes to reach the end point,what is the molarity sulfide in the water sample?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
A current of 0.500 A flows through a cell containing Fe2+ for 10.0 minutes.Calculate the maximum moles of Fe that can be removed from solution? Assume constant current over time.
A)1)04 mmol
B)51.8 mol
C)3)11 mmol
D)1)55 mmol
E)25.9 mol
A)1)04 mmol
B)51.8 mol
C)3)11 mmol
D)1)55 mmol
E)25.9 mol

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
____________________ measures the electric current flowing between a pair of electrodes driving an electrolysis reaction.The amount of current is proportional to the concentration of the analyte.
A)Square wave voltametry
B)Polarography
C)Cyclic Voltametry
D)Amperometry
E)Coulometry
A)Square wave voltametry
B)Polarography
C)Cyclic Voltametry
D)Amperometry
E)Coulometry

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
During electrolysis,electrons flow from the____________________ terminal of the power supply into the____________________of the electrolysis cell.Electrons flow from the____________________ into the____________________ terminal of the power supply to complete the circuit.
A)negative;anode;ground;positive
B)positive;cathode;anode;negative
C)negative;ground;cathode;positive
D)negative;cathode;anode;positive
E)positive;anode;cathode;negative
A)negative;anode;ground;positive
B)positive;cathode;anode;negative
C)negative;ground;cathode;positive
D)negative;cathode;anode;positive
E)positive;anode;cathode;negative

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Electrolysis of 200 mL of a solution of an unknown metal increased the cathode mass by 0.0434 grams when a current of 0.50 A passed through the cell for 5 minutes.Further research determined the charge on the metal to be +2.What is the identity of the metal?
A)Rg
B)Si
C)Fe
D)Cd
E)Mn
A)Rg
B)Si
C)Fe
D)Cd
E)Mn

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Sodium sulfate cells generate hydrogen and oxygen gas when a current is passed through the cell.For the following data,calculate the potential required for the cell to generate partial pressures of 0.25 atm for oxygen and 0.50 atm for hydrogen.At the cathode surface the pH is 10 and at the anode surface the pH is 4.A current of 0.32 amps passes through the solution,which has a resistance of 3 .The overpotentials for the anode and cathode are 0.580 V and 0.584 V respectively.
O2 + 4 H+ + 4 e- ⇋ 2 H2O Eo = 1.2291 V
2 H2O + 2 e- ⇋ H2 + 2 OH- Eo = -0.8280 V
O2 + 4 H+ + 4 e- ⇋ 2 H2O Eo = 1.2291 V
2 H2O + 2 e- ⇋ H2 + 2 OH- Eo = -0.8280 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statement(s)is(are)TRUE for coulometry?
I Coulometric methods count electrons used in a chemical reaction to measure analyte.
II Coulometry employs either constant-current or constant-potential conditions.
III An advantage of coulometric methods is high precision and a disadvantage is low sensitivity.
IV Coulometric titrations are constant-potential methods.
V Constant-potential methods utilizing a three-electrode cell are more selective than constant-current methods.
A)I,II,and V
B)I,II,IV and V
C)I,III,and IV
D)II,IV,and V
E)I and II
I Coulometric methods count electrons used in a chemical reaction to measure analyte.
II Coulometry employs either constant-current or constant-potential conditions.
III An advantage of coulometric methods is high precision and a disadvantage is low sensitivity.
IV Coulometric titrations are constant-potential methods.
V Constant-potential methods utilizing a three-electrode cell are more selective than constant-current methods.
A)I,II,and V
B)I,II,IV and V
C)I,III,and IV
D)II,IV,and V
E)I and II

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


