Deck 22: Atomic Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 22: Atomic Spectroscopy
1
All of the following are true for hollow-cathode tubes,except:
A)hollow-cathode tubes emit narrow emission lines,too narrow for slits to isolate.
B)the cathode of a hollow-cathode tube is made of the same element whose emission lines we want.
C)the low pressure Ne or Ar gas is ionized when voltage is applied,which ionizes the gas and accelerates the ions toward the cathode.
D)the kinetic energy of the ionized gas is sufficiently high to sputter metal atoms from the cathode.
E)the gaseous metal atoms are excited by collisions with high-energy electrons and emit photons with characteristic wavelengths that are absorbed by the analyte.
A)hollow-cathode tubes emit narrow emission lines,too narrow for slits to isolate.
B)the cathode of a hollow-cathode tube is made of the same element whose emission lines we want.
C)the low pressure Ne or Ar gas is ionized when voltage is applied,which ionizes the gas and accelerates the ions toward the cathode.
D)the kinetic energy of the ionized gas is sufficiently high to sputter metal atoms from the cathode.
E)the gaseous metal atoms are excited by collisions with high-energy electrons and emit photons with characteristic wavelengths that are absorbed by the analyte.
hollow-cathode tubes emit narrow emission lines,too narrow for slits to isolate.
2
X-rays are generated when:
A)metals such as W,Mo,Ag,or Rh are heated to high temperatures.
B)high energy photons strike a metal such as W,Mo,Ag,or Rh.
C)high-energy electrons strike an anode made of metals such as W,Mo,Ag,or Rh.
D)high-energy protons strike an anode made of metals such as W,Mo,Ag,or Rh.
E)metals such as W,Mo,Ag,or Rh are electrically excited.
A)metals such as W,Mo,Ag,or Rh are heated to high temperatures.
B)high energy photons strike a metal such as W,Mo,Ag,or Rh.
C)high-energy electrons strike an anode made of metals such as W,Mo,Ag,or Rh.
D)high-energy protons strike an anode made of metals such as W,Mo,Ag,or Rh.
E)metals such as W,Mo,Ag,or Rh are electrically excited.
high-energy electrons strike an anode made of metals such as W,Mo,Ag,or Rh.
3
Which is NOT true of the flame atomic absorbance spectroscopy of iron?
A)The iron sample is aspirated into the flame,where the solvent evaporates and the iron analyte is atomized.
B)The flame replaces the cuvet of conventional spectrometers and the flame pathlength is typically 10 cm.
C)The hollow-cathode lamp for iron emits wavelengths of light unique for iron.
D)The iron atoms in the flame absorb some of the light emitted from the hollow-cathode lamp.
E)A detector measures the amount of light generated by the flame and not absorbed by iron.
A)The iron sample is aspirated into the flame,where the solvent evaporates and the iron analyte is atomized.
B)The flame replaces the cuvet of conventional spectrometers and the flame pathlength is typically 10 cm.
C)The hollow-cathode lamp for iron emits wavelengths of light unique for iron.
D)The iron atoms in the flame absorb some of the light emitted from the hollow-cathode lamp.
E)A detector measures the amount of light generated by the flame and not absorbed by iron.
A detector measures the amount of light generated by the flame and not absorbed by iron.
4
Which of the following statements are true of atomic emission spectroscopy (AES)?
I Atoms are promoted to an excited state with a laser.
II Atoms are promoted to an excited state by gaining energy from collisions with other atoms or from the high thermal energy of the flame.
III The intensity of emitted light is proportional to analyte concentration.
IV Plasma often replaces flame in AES.
A)III and IV
B)I,III,and IV
C)II,III,and IV
D)II and III
E)I and III
I Atoms are promoted to an excited state with a laser.
II Atoms are promoted to an excited state by gaining energy from collisions with other atoms or from the high thermal energy of the flame.
III The intensity of emitted light is proportional to analyte concentration.
IV Plasma often replaces flame in AES.
A)III and IV
B)I,III,and IV
C)II,III,and IV
D)II and III
E)I and III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
To measure calcium in a breakfast cereal,0.6590 grams of crushed cereal were reacted for 1 hour in warm 1 M HNO3.The reaction mixture was filtered and the filtrate transferred to a 100 mL volumetric flask.A series of eight standards are prepared by transferring 5.00 mL aliquots to 50-mL volumetric flasks to which increasing volumes of standard Ca2+ (containing 20.0 g/mL)is added,diluting to the final volume with distilled deionized H2O.The samples were then analyzed by flame atomic absorption.Use the data and graph below to find the percent by mass calcium in the cereal.
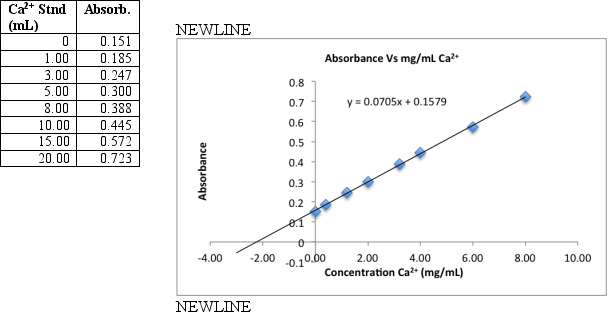


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
The dynamic reaction cell reduces isobaric interference with thermodynamically favorable reactions that remove interfering species with the same mass-to-charge ratio.Which of the reactions does not remove interfering species for 52Cr+ or 56Fe2+?
A)(40Ar12C+ + NH3 →
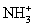
+ Ar + C)
B)(40Ar16O+ + NH3 →
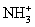
+ Ar + O)
C)(35Cl16OH+ + NH3 →
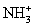
+ ClO)
D)(40Ca16O+ + CO → Ca+ + CO2)
E)(35Cl17OH+ + NH3 →
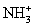
+ ClO)
A)(40Ar12C+ + NH3 →
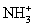
+ Ar + C)
B)(40Ar16O+ + NH3 →
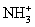
+ Ar + O)
C)(35Cl16OH+ + NH3 →
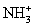
+ ClO)
D)(40Ca16O+ + CO → Ca+ + CO2)
E)(35Cl17OH+ + NH3 →
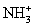
+ ClO)

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Graphite furnace employs temperature programming to produce elemental vapor.The program begins by raising the furnace temperature to initiate____________________ ,where water is removed from the sample.Next,the temperature of the furnace is then raised to begin the____________________ process,where organic material is removed.The temperature of the furnace is raised again to____________________ the sample,creating the elemental vapor required for spectroscopy.
A)baking;desiccation;atomize
B)drying;desiccation;fragment
C)drying;charring;atomize
D)drying;atomize;charring
E)baking;atomize;desiccation
A)baking;desiccation;atomize
B)drying;desiccation;fragment
C)drying;charring;atomize
D)drying;atomize;charring
E)baking;atomize;desiccation

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
There is no universal flame temperature for flame-based atomic spectroscopy that gives optimal results for all elements.________________________________________gives a____________________.
A)Excess oxidant;cooler flame
B)Excess oxidant;hotter flame
C)Shorter aspiration time;cooler flame
D)Longer aspiration time;hotter flame
E)Excess analyte;hotter flame
A)Excess oxidant;cooler flame
B)Excess oxidant;hotter flame
C)Shorter aspiration time;cooler flame
D)Longer aspiration time;hotter flame
E)Excess analyte;hotter flame

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Inductively coupled argon plasma does not suffer from many of the interferences encountered with flame atomic spectroscopy.Which of the statements below are NOT advantages of plasma over flame?
A)Plasma is twice as hot as conventional flame.
B)Formation of analyte oxides and hydroxides is negligible.
C)Atomization is more complete and signal is enhanced.
D)Analyte in plasma self-absorbs at a higher rate than in flame.
E)Analyte residence time in the plasma is about twice as long as in flame.
A)Plasma is twice as hot as conventional flame.
B)Formation of analyte oxides and hydroxides is negligible.
C)Atomization is more complete and signal is enhanced.
D)Analyte in plasma self-absorbs at a higher rate than in flame.
E)Analyte residence time in the plasma is about twice as long as in flame.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
____________________ is any effect that changes the signal while analyte concentration remains unchanged.
A)Scattering
B)Interference
C)Self-absorption
D)Diffraction
E)Refraction
A)Scattering
B)Interference
C)Self-absorption
D)Diffraction
E)Refraction

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements are true regarding the effect of flame temperature on atomic absorbance and atomic emission spectroscopy?
I Varying the flame temperature by 10 K hardly affects the ground-state population of the analyte and would not noticeably affect the analyte signal in atomic absorption.
II Varying the flame temperature by 10 K hardly affects the excited-state population of the analyte and would not noticeably affect the analyte signal in atomic emission.
III Varying the flame temperature by 10 K affects the ground-state population of the analyte and will noticeably affect the analyte signal in atomic absorption.
IV Varying the flame temperature by 10 K affects the excited-state population of the analyte and will noticeably affect the analyte signal in atomic emission.
A)I and II
B)I and IV
C)II and III
D)III and IV
E)II and IV
I Varying the flame temperature by 10 K hardly affects the ground-state population of the analyte and would not noticeably affect the analyte signal in atomic absorption.
II Varying the flame temperature by 10 K hardly affects the excited-state population of the analyte and would not noticeably affect the analyte signal in atomic emission.
III Varying the flame temperature by 10 K affects the ground-state population of the analyte and will noticeably affect the analyte signal in atomic absorption.
IV Varying the flame temperature by 10 K affects the excited-state population of the analyte and will noticeably affect the analyte signal in atomic emission.
A)I and II
B)I and IV
C)II and III
D)III and IV
E)II and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Fluctuations in flame temperature affect the reproducibility of atomic emission experiments more so than atomic absorbance experiments.Which of the following statements explain this phenomenon?
I As temperature increases there is an increase in emission intensity resulting from an increase in the excited state population;the apparent concentration is greater than the actual concentration.
II As temperature decreases there is a decrease in emission intensity resulting from an increase in the excited state population;the apparent concentration is greater than the actual concentration.
III As temperature increases there is a decrease in emission intensity resulting from a decrease in the excited state population;the apparent concentration is less than the actual concentration.
IV As temperature decreases there is a decrease in emission intensity resulting from a decrease in the excited state population;the apparent concentration is less than the actual concentration.
A)II and III
B)I and IV
C)I and III
D)II and IV
E)None of these statements explains this phenomenon.
I As temperature increases there is an increase in emission intensity resulting from an increase in the excited state population;the apparent concentration is greater than the actual concentration.
II As temperature decreases there is a decrease in emission intensity resulting from an increase in the excited state population;the apparent concentration is greater than the actual concentration.
III As temperature increases there is a decrease in emission intensity resulting from a decrease in the excited state population;the apparent concentration is less than the actual concentration.
IV As temperature decreases there is a decrease in emission intensity resulting from a decrease in the excited state population;the apparent concentration is less than the actual concentration.
A)II and III
B)I and IV
C)I and III
D)II and IV
E)None of these statements explains this phenomenon.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Hollow-cathode lamps emit light with very sharp linewidths.Which mechanisms are responsible for broadening atomic emission line widths?
I pressure broadening
II ionization effect
III matrix effect
IV Doppler effect
A)I and IV
B)II,III,and IV
C)I,III,and IV
D)II and IV
E)III and IV
I pressure broadening
II ionization effect
III matrix effect
IV Doppler effect
A)I and IV
B)II,III,and IV
C)I,III,and IV
D)II and IV
E)III and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements are true for chemical interference?
I Releasing agents are added to the sample to reduce chemical interference.
II Chemical interference occurs when a component of the sample reduces the extent of atomization of the analyte.
III Higher temperature flames will reduce chemical interference.
IV Chemical interference occurs when a component of the sample emits light at or close to the same frequency as the analyte.
V Releasing agents will preferentially react with the analyte,protecting the analyte from components of the sample that will ionize the analyte.
A)III,IV,and V
B)I,III,and IV
C)I,II,and IV
D)I,II,and III
E)II,III,and V
I Releasing agents are added to the sample to reduce chemical interference.
II Chemical interference occurs when a component of the sample reduces the extent of atomization of the analyte.
III Higher temperature flames will reduce chemical interference.
IV Chemical interference occurs when a component of the sample emits light at or close to the same frequency as the analyte.
V Releasing agents will preferentially react with the analyte,protecting the analyte from components of the sample that will ionize the analyte.
A)III,IV,and V
B)I,III,and IV
C)I,II,and IV
D)I,II,and III
E)II,III,and V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
____________________ occurs for inductively coupled plasma-mass spectroscopy when the interfering species has the same mass-to-charge ratio as the analyte ion.
A)Ionization interference
B)Analyte interference
C)Isobaric interference
D)Allotrope interference
E)Isothermal interference
A)Ionization interference
B)Analyte interference
C)Isobaric interference
D)Allotrope interference
E)Isothermal interference

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is NOT true for X-ray fluorescence?
A)The fluorescent X-ray photon has a lower energy than the original X-ray photon.
B)The incoming X-ray knocks free an electron from the K,L,or M shells.
C)The fluorescent X-ray photon is emitted when an outer shell electron falls into a shell closer to the nucleus.
D)K X-rays original when L or M shell electrons fall into the K shell.
E)K and K X-rays originate when L shell electrons transition to the K shell.
A)The fluorescent X-ray photon has a lower energy than the original X-ray photon.
B)The incoming X-ray knocks free an electron from the K,L,or M shells.
C)The fluorescent X-ray photon is emitted when an outer shell electron falls into a shell closer to the nucleus.
D)K X-rays original when L or M shell electrons fall into the K shell.
E)K and K X-rays originate when L shell electrons transition to the K shell.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
The energy difference between the ground and excited state and temperature has the following effect on the excited-state population: As the energy difference between the ground state and excited state decreases,the excited-state population________________________________________.As the temperature of the flame increases,the excited-state population____________________ .
A)remains the same;increases
B)decreases;decreases
C)increases;remains the same
D)decreases;increases
E)decreases;remains the same
A)remains the same;increases
B)decreases;decreases
C)increases;remains the same
D)decreases;increases
E)decreases;remains the same

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is(are)NOT true for ionization interference?
I Ionization interference can occur with alkali metals at relatively low temperature and for analyses for other elements at higher temperature.
II Ionization suppressors decrease the extent of ionization of analyte.
III Ionization suppressors are more easily ionized than the analyte,the large number of electrons produced prevent ionization of the analyte.
IV Ionization interference occurs when a component of the sample prevents ionization of the sample.
A)IV
B)II
C)I and II
D)III
E)III and IV
I Ionization interference can occur with alkali metals at relatively low temperature and for analyses for other elements at higher temperature.
II Ionization suppressors decrease the extent of ionization of analyte.
III Ionization suppressors are more easily ionized than the analyte,the large number of electrons produced prevent ionization of the analyte.
IV Ionization interference occurs when a component of the sample prevents ionization of the sample.
A)IV
B)II
C)I and II
D)III
E)III and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
All of the following are background correction techniques for atomic spectroscopy,except:
A)subtraction of adjacent pixel height from signal pixel height for a charge injection detector.
B)a rotating beam chopper periodically blocks the analyte signal,allowing for a background measurement.
C)the absorbance and scattering of the light from a D2 lamp is measured and subtracted from the absorbance and scattering of light from a hollow-cathode tube.
D)the analyte sample is spiked with a compound to absorb all background signal.
E)a strong magnetic field is pulsed on and off.The sample and background is observed when field off.Only the background is observed when the field is on.The absorbance difference is the analyte signal.
A)subtraction of adjacent pixel height from signal pixel height for a charge injection detector.
B)a rotating beam chopper periodically blocks the analyte signal,allowing for a background measurement.
C)the absorbance and scattering of the light from a D2 lamp is measured and subtracted from the absorbance and scattering of light from a hollow-cathode tube.
D)the analyte sample is spiked with a compound to absorb all background signal.
E)a strong magnetic field is pulsed on and off.The sample and background is observed when field off.Only the background is observed when the field is on.The absorbance difference is the analyte signal.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Laser ablation is an inductively coupled plasma sampling technique.Which of the following is true for laser ablation?
I Laser ablation destroys the entire sample.
II A laser pulse is focused onto a small area,producing an explosion of atoms,electrons,and ions in the gas phase.
III Depth profiling of the sample is possible with repeated laser pulses probing deeper and deeper into the sample.
IV Nanogram amounts of material are ablated per pulse.
V Ablation products are swept to the plasma with H2 gas.
A)I and II
B)II,III,and IV
C)I,II,III,and IV
D)II,IV,and V
E)III and IV
I Laser ablation destroys the entire sample.
II A laser pulse is focused onto a small area,producing an explosion of atoms,electrons,and ions in the gas phase.
III Depth profiling of the sample is possible with repeated laser pulses probing deeper and deeper into the sample.
IV Nanogram amounts of material are ablated per pulse.
V Ablation products are swept to the plasma with H2 gas.
A)I and II
B)II,III,and IV
C)I,II,III,and IV
D)II,IV,and V
E)III and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


