Deck 3: Measurement and Chemical Calculations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 3: Measurement and Chemical Calculations
1
A 60-kg astronaut travels from the earth to the moon.If the gravitational attraction on the moon is 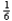 of that on earth,what will be her mass on the moon?
of that on earth,what will be her mass on the moon?
A)0 kg
B)10 kg
C)60 kg
D)360 kg
E)3600 kg
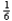 of that on earth,what will be her mass on the moon?
of that on earth,what will be her mass on the moon?A)0 kg
B)10 kg
C)60 kg
D)360 kg
E)3600 kg
60 kg
2
Complete the following operation: 8.36 × 106 + 1.320 × 107
A)8.492 × 107
B)8.492 × 108
C)9.68 × 1013
D)2.156 × 108
E)2.156 × 107
A)8.492 × 107
B)8.492 × 108
C)9.68 × 1013
D)2.156 × 108
E)2.156 × 107
2.156 × 107
3
Which of the following is/are metric units of mass?
i.Pound
ii.Gram
iii.Ounce
iv.Kilogram
v.Centigram
A)ii only
B)iv only
C)ii and iv
D)i and iii
E)ii, iv, and v
i.Pound
ii.Gram
iii.Ounce
iv.Kilogram
v.Centigram
A)ii only
B)iv only
C)ii and iv
D)i and iii
E)ii, iv, and v
ii and iv
4
Complete the following operation: 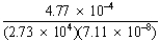
A)4.07
B)0.246
C)2.46 × 10-6
D)1.24 × 10-4
E)1.24 × 10-15
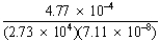
A)4.07
B)0.246
C)2.46 × 10-6
D)1.24 × 10-4
E)1.24 × 10-15

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
Convert 1.57 g to kg,cg,and mg.
A)1570 kg, 0.0157 cg, 0.00157 mg
B)0.00157 kg, 0.0157 cg, 1570 mg
C)0.00157 kg, 157 cg, 1570 mg
D)157 kg, 1570 cg, 0.00157 mg
E)1570 kg, 0.00157 cg, 157 mg
A)1570 kg, 0.0157 cg, 0.00157 mg
B)0.00157 kg, 0.0157 cg, 1570 mg
C)0.00157 kg, 157 cg, 1570 mg
D)157 kg, 1570 cg, 0.00157 mg
E)1570 kg, 0.00157 cg, 157 mg

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
Consider a situations in which where you are walking at 3.7 miles per hour.Which of the following is true of the Per expression given in this statement?
A)3.7 mi = 1 hr
B)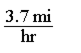
C)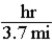
D)Distance in miles is directly proportional to time in hours.
E)All of these are true for the given Per expression.
A)3.7 mi = 1 hr
B)
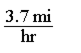
C)
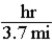
D)Distance in miles is directly proportional to time in hours.
E)All of these are true for the given Per expression.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following numbers are correctly expressed in ordinary decimal form?
i.6.21 × 105 = 621,000
ii.7.28 × 106 = 0.00000728
iii.9.03 × 10-4 = 0.000903
iv.1.12 × 10-3 = 1120
A)i and ii
B)i and iii
C)ii and iv
D)iii and iv
E)All are incorrect
i.6.21 × 105 = 621,000
ii.7.28 × 106 = 0.00000728
iii.9.03 × 10-4 = 0.000903
iv.1.12 × 10-3 = 1120
A)i and ii
B)i and iii
C)ii and iv
D)iii and iv
E)All are incorrect

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
Convert 9.16 kg to Gg,hg,and µg.1 Gg = 109 g,1 hg = 102 g,1 µg = 10-6 g
A)9.16 × 1012 Gg, 9.16 × 105 hg, 9.16 × 10-3 µg
B)9.16 × 10-12 Gg, 9.16 × 10-5 hg, 9.16 × 103 µg
C)9.16 × 106 Gg, 9.16 × 10-1 hg, 9.16 × 10-9 µg
D)9.16 × 10-6 Gg, 9.16 × 101 hg, 9.16 × 109 µg
E)9.16 × 10-9 Gg, 9.16 × 10-2 hg, 9.16 × 106 µg
A)9.16 × 1012 Gg, 9.16 × 105 hg, 9.16 × 10-3 µg
B)9.16 × 10-12 Gg, 9.16 × 10-5 hg, 9.16 × 103 µg
C)9.16 × 106 Gg, 9.16 × 10-1 hg, 9.16 × 10-9 µg
D)9.16 × 10-6 Gg, 9.16 × 101 hg, 9.16 × 109 µg
E)9.16 × 10-9 Gg, 9.16 × 10-2 hg, 9.16 × 106 µg

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following numbers are correctly expressed in exponential notation?
i.43,200,000 = 4.32 × 107
ii.0.000977 = 9.77 × 104
iii.606,000 = 6.06 × 10-5
iv.0.00000175 = 1.75 × 10-6
A)i and iv
B)ii and iii
C)i and iii
D)ii and iv
E)All are incorrect
i.43,200,000 = 4.32 × 107
ii.0.000977 = 9.77 × 104
iii.606,000 = 6.06 × 10-5
iv.0.00000175 = 1.75 × 10-6
A)i and iv
B)ii and iii
C)i and iii
D)ii and iv
E)All are incorrect

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
How many fluid ounces are in a 2.5 gallon container? By definition,there are 4 qt/gal and 32 fl oz/qt.
A)0.0031 fl oz
B)0.31 fl oz
C)0.020 fl oz
D)20 fl oz
E)3.2 × 102 fl oz
A)0.0031 fl oz
B)0.31 fl oz
C)0.020 fl oz
D)20 fl oz
E)3.2 × 102 fl oz

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
What will be the cost in dollars for photocopies if you have 32 pages in a booklet,you need 175 booklets,and copies are 4.5 cents each?
A)$12.44
B)$24.61
C)$39.68
D)$252
E)$25,200
A)$12.44
B)$24.61
C)$39.68
D)$252
E)$25,200

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
Convert 275 kg to g,cg,and mg.
A)2.75 × 10-1 g, 2.75 × 10-3 cg, 2.75 × 10-4 mg
B)2.75 ×10-1 g, 2.75 × 101 cg, 2.75 × 102 mg
C)2.75 × 105 g, 2.75 × 103 cg, 2.75 × 102 mg
D)2.75 × 105 g, 2.75 × 108 cg, 2.75 × 107 mg
E)2.75 × 105 g, 2.75 × 107 cg, 2.75 × 108 mg
A)2.75 × 10-1 g, 2.75 × 10-3 cg, 2.75 × 10-4 mg
B)2.75 ×10-1 g, 2.75 × 101 cg, 2.75 × 102 mg
C)2.75 × 105 g, 2.75 × 103 cg, 2.75 × 102 mg
D)2.75 × 105 g, 2.75 × 108 cg, 2.75 × 107 mg
E)2.75 × 105 g, 2.75 × 107 cg, 2.75 × 108 mg

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
Convert 32.7 m to km,cm,and mm.
A)0.0327 km, 3270 cm, 32,700 mm
B)3270 km, 32,700 cm, 0.0327 mm
C)32,700 km, 0.0327 cm, 3270 mm
D)32,700 km, 0.327 cm, 0.0327 mm
E)32,700 km, 0.0327 cm, 0.327 mm
A)0.0327 km, 3270 cm, 32,700 mm
B)3270 km, 32,700 cm, 0.0327 mm
C)32,700 km, 0.0327 cm, 3270 mm
D)32,700 km, 0.327 cm, 0.0327 mm
E)32,700 km, 0.0327 cm, 0.327 mm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
Convert 17.4 nm to Mm,Gm,and Tm.1 nm = 10-9 m,1 Mm = 106 m,1 Gm = 109 m,1 Tm = 1012 m
A)1.74 × 10-14 Mm, 1.74 × 10-17 Gm, 1.74 × 10-20 Tm
B)1.74 × 10-16 Mm, 1.74 ×10-19 Gm, 1.74 × 10-22 Tm
C)1.74 × 1016 Mm, 1.74 × 1019 Gm, 1.74 × 1022 Tm
D)1.74 × 1018 Mm, 1.74 ×1021 Gm, 1.74 × 1024 Tm
E)1.74 × 104 Mm, 1.74 × 101 Gm, 1.74 × 10-2 Tm
A)1.74 × 10-14 Mm, 1.74 × 10-17 Gm, 1.74 × 10-20 Tm
B)1.74 × 10-16 Mm, 1.74 ×10-19 Gm, 1.74 × 10-22 Tm
C)1.74 × 1016 Mm, 1.74 × 1019 Gm, 1.74 × 1022 Tm
D)1.74 × 1018 Mm, 1.74 ×1021 Gm, 1.74 × 1024 Tm
E)1.74 × 104 Mm, 1.74 × 101 Gm, 1.74 × 10-2 Tm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following numbers are correctly expressed in both exponential notation and ordinary decimal form?
i.4.09 × 10-3 = 4090
ii.3.72 × 10-5 = 0.0000372
iii.62,800 = 6.28 × 104
iv.5,910,000 = 5.91 × 10-6
A)i and ii
B)i and iii
C)ii and iii
D)iii and iv
E)All are incorrect
i.4.09 × 10-3 = 4090
ii.3.72 × 10-5 = 0.0000372
iii.62,800 = 6.28 × 104
iv.5,910,000 = 5.91 × 10-6
A)i and ii
B)i and iii
C)ii and iii
D)iii and iv
E)All are incorrect

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is/are metric units of volume?
i.mL
ii.L
iii.cm3
iv.m
v.kg
A)i and ii
B)i, ii, and iii
C)ii only
D)iv and v
E)All are metric units of volume
i.mL
ii.L
iii.cm3
iv.m
v.kg
A)i and ii
B)i, ii, and iii
C)ii only
D)iv and v
E)All are metric units of volume

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
Convert 373 liters to milliliters,centiliters,and kiloliters.
A)0.373 mL, 373,000 cL, 37,300 kL
B)37,300 mL, 0.373 cL, 373,000 kL
C)373,000 mL, 37,300 cL, 0.373 kL
D)0.373 mL, 3.73 cL, 373,000 kL
E)3.73 mL, 0.373 cL, 373,000 kL
A)0.373 mL, 373,000 cL, 37,300 kL
B)37,300 mL, 0.373 cL, 373,000 kL
C)373,000 mL, 37,300 cL, 0.373 kL
D)0.373 mL, 3.73 cL, 373,000 kL
E)3.73 mL, 0.373 cL, 373,000 kL

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
Complete the following operation: 9.370 × 10-5 - 2.25 × 10-6
A)7.12 × 101
B)7.12 × 10-11
C)-9.145 × 10-5
D)9.145 × 10-5
E)9.145 × 10-4
A)7.12 × 101
B)7.12 × 10-11
C)-9.145 × 10-5
D)9.145 × 10-5
E)9.145 × 10-4

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following would be the best unit to measure the mass of a molecule of sugar?
A)pg
B)Mg
C)kg
D)g
E)mg
A)pg
B)Mg
C)kg
D)g
E)mg

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
Convert 3.77 × 108 pL to mL,daL,and dL.1 pL = 10-12 L,1 dL = 10-1 L,1 daL = 101 L
A)3.77 × 1023 mL, 3.77 × 1019 daL, 3.77 × 1021 dL
B)3.77 × 1017 mL, 3.77 × 1021 daL, 3.77 × 1019 dL
C)3.77 × 10-1 mL, 3.77 × 10-5 daL, 3.77 × 10-3 dL
D)3.77 × 10-4 mL, 3.77 × 10-5 daL, 3.77 × 10-3 dL
E)3.77 × 10-7 mL, 3.77 × 10-3 daL, 3.77 × 10-5 dL
A)3.77 × 1023 mL, 3.77 × 1019 daL, 3.77 × 1021 dL
B)3.77 × 1017 mL, 3.77 × 1021 daL, 3.77 × 1019 dL
C)3.77 × 10-1 mL, 3.77 × 10-5 daL, 3.77 × 10-3 dL
D)3.77 × 10-4 mL, 3.77 × 10-5 daL, 3.77 × 10-3 dL
E)3.77 × 10-7 mL, 3.77 × 10-3 daL, 3.77 × 10-5 dL

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
Examine the following graph. 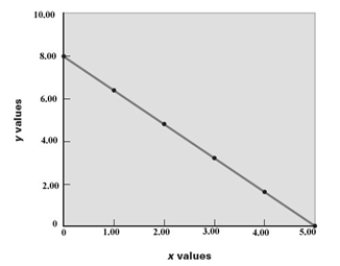 What is the value of x when y = 2.00?
What is the value of x when y = 2.00?
A)2.00
B)3.75
C)11.3
D)5.00
E)4.00
 What is the value of x when y = 2.00?
What is the value of x when y = 2.00?A)2.00
B)3.75
C)11.3
D)5.00
E)4.00

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
Consider that 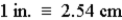 .If 1559 cm is converted to inches,how many significant figures should be in the answer?
.If 1559 cm is converted to inches,how many significant figures should be in the answer?
A)1
B)2
C)3
D)4
E)5
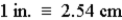 .If 1559 cm is converted to inches,how many significant figures should be in the answer?
.If 1559 cm is converted to inches,how many significant figures should be in the answer?A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the three thermometers shown in the image. 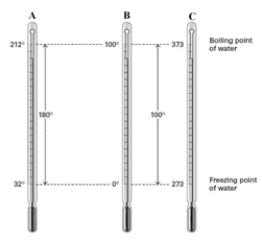 Which of the following equations correctly represents the equation relating reading on the A thermometer to the B thermometer?
Which of the following equations correctly represents the equation relating reading on the A thermometer to the B thermometer?
A)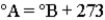
B)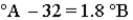
C)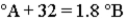
D)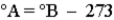
E)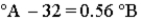
 Which of the following equations correctly represents the equation relating reading on the A thermometer to the B thermometer?
Which of the following equations correctly represents the equation relating reading on the A thermometer to the B thermometer?A)
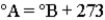
B)
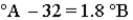
C)
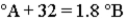
D)
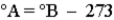
E)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
A solution is prepared by adding 1.77 grams of sodium nitrate,2.4 grams of potassium chloride,and 0.973 gram of ammonium nitrite to 255 grams of water.Calculate the total mass of the solution and express the sum in the proper number of significant figures.
A)2.6 × 102 g
B)2.60 × 102 g
C)2.601 × 102 g
D)2.6014 × 102 g
E)2.60143 × 102 g
A)2.6 × 102 g
B)2.60 × 102 g
C)2.601 × 102 g
D)2.6014 × 102 g
E)2.60143 × 102 g

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
The meter stick in the image is being used to measure the length of a piece of wood. 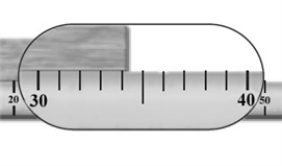 How many significant figures should be used to express this measured length?
How many significant figures should be used to express this measured length?
A)1
B)2
C)3
D)4
E)5
 How many significant figures should be used to express this measured length?
How many significant figures should be used to express this measured length?A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the best mathematical expression to represent the fact that the pressure of a gas (P),at constant volume and temperature,is directly proportional to the mass of that gas (m)?
A)P ∝ m
B)P = m
C)Pm
D)P ≈ m
E)P ∈ m
A)P ∝ m
B)P = m
C)Pm
D)P ≈ m
E)P ∈ m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
How many significant figures are in each of the following quantities?
i. 0.00062 kg
ii. 0.720 in.
iii. 4.150 × 103 lb
iv. 0.305 m3
A) i. 2 ii. 2 iii. 3 iv. 3
B) i. 2 ii. 2 iii. 4 iv. 3
C) i. 5 ii. 3 iii. 4 iv. 3
D) i. 2 ii. 3 iii. 4 iv. 3.
E) i. 5 ii. 3 iii. 4 iv. 3.
i. 0.00062 kg
ii. 0.720 in.
iii. 4.150 × 103 lb
iv. 0.305 m3
A) i. 2 ii. 2 iii. 3 iv. 3
B) i. 2 ii. 2 iii. 4 iv. 3
C) i. 5 ii. 3 iii. 4 iv. 3
D) i. 2 ii. 3 iii. 4 iv. 3.
E) i. 5 ii. 3 iii. 4 iv. 3.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
Faraday's Law of Electrolysis states that the mass of a substance produced at an electrode during electrolysis (m)is directly proportional to the number of moles of electrons transferred at that electrode (n).Which of the following equations correctly characterizes the relation?
A)m = k × n
B)As the number of electrons transferred increases the mass deposited decreases.
C)There is one conversion factor possible that relates m and n.
D)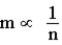
E)All of the above correctly describe this relation.
A)m = k × n
B)As the number of electrons transferred increases the mass deposited decreases.
C)There is one conversion factor possible that relates m and n.
D)
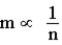
E)All of the above correctly describe this relation.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
Acetone boils at 56 °C.Express this temperature in kelvins.
A)-329 K
B)-217 K
C)133 K
D)217 K
E)329 K
A)-329 K
B)-217 K
C)133 K
D)217 K
E)329 K

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following measured number.640,000 m If this measurement were made to the nearest km (± 1 km),how should the answer be expressed in exponential notation?
A)6.4 × 105 m
B)6.40 × 105 m
C)6.400 × 105 m
D)6.4000 × 105 m
E)6.40000 × 105 m
A)6.4 × 105 m
B)6.40 × 105 m
C)6.400 × 105 m
D)6.4000 × 105 m
E)6.40000 × 105 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
A filled shipping box weighs 12 lb.How many kilograms is this? 1 lb = 453.59237 g
A)5.4 kg
B)5.4 × 103 kg
C)5.4 × 106 kg
D)26 kg
E)2.6 × 10-5 kg
A)5.4 kg
B)5.4 × 103 kg
C)5.4 × 106 kg
D)26 kg
E)2.6 × 10-5 kg

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
The melting point of iron is 1535.0°C.What is the Fahrenheit equivalent of this temperature?
A)821.0°F
B)885.0°F
C)2731.0°F
D)2795.0°F
E)2821.0°F
A)821.0°F
B)885.0°F
C)2731.0°F
D)2795.0°F
E)2821.0°F

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
The temperature in the laboratory is posted as 295 K.What is this temperature in degrees Celsius?
A)22 °C
B)-22 °C
C)568 °C
D)-568 °C
E)563 °C
A)22 °C
B)-22 °C
C)568 °C
D)-568 °C
E)563 °C

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
Convert 5.01 × 103 cm to km,m,and mm.
A)5.01 × 10-2 km, 5.01 × 101 m, 5.01 × 104 mm
B)5.01 × 10-2 km, 5.01 × 101 m, 5.01 × 103 mm
C)5.01 × 102 km, 5.01 × 105 m, 5.01 × 108 mm
D)5.01 × 104 km, 5.01 × 101 m, 5.01 × 10-2 mm
E)5.01 × 108 km, 5.01 × 105 m, 5.01 × 102 mm
A)5.01 × 10-2 km, 5.01 × 101 m, 5.01 × 104 mm
B)5.01 × 10-2 km, 5.01 × 101 m, 5.01 × 103 mm
C)5.01 × 102 km, 5.01 × 105 m, 5.01 × 108 mm
D)5.01 × 104 km, 5.01 × 101 m, 5.01 × 10-2 mm
E)5.01 × 108 km, 5.01 × 105 m, 5.01 × 102 mm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following graph. 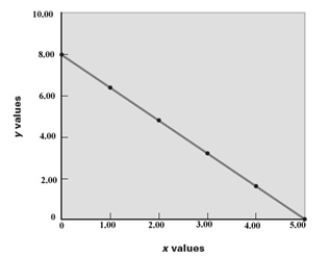 For the relationship shown on the graph,what are the values of m and b,respectively,in the equation,y = mx + b?
For the relationship shown on the graph,what are the values of m and b,respectively,in the equation,y = mx + b?
A)8.00, 5.00
B)8.00, -1.60
C)1.60, 5.00
D)0.625, 8.00
E)-1.60, 8.00
 For the relationship shown on the graph,what are the values of m and b,respectively,in the equation,y = mx + b?
For the relationship shown on the graph,what are the values of m and b,respectively,in the equation,y = mx + b?A)8.00, 5.00
B)8.00, -1.60
C)1.60, 5.00
D)0.625, 8.00
E)-1.60, 8.00

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
The Willis Tower in Chicago is 1451 ft tall.How high is this in meters? 1 in.= 2.54 cm
A)68.55 m
B)0.4760 m
C)4.423 × 106 m
D)4.423 × 104 m
E)442.3 m
A)68.55 m
B)0.4760 m
C)4.423 × 106 m
D)4.423 × 104 m
E)442.3 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
Assuming that all are measured quantities,complete the following operation and express the result in the correct number of significant figures. 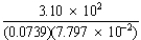
A)3.27 × 102
B)3.271 × 102
C)5.4 × 104
D)5.38 × 104
E)5.380 × 104
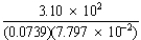
A)3.27 × 102
B)3.271 × 102
C)5.4 × 104
D)5.38 × 104
E)5.380 × 104

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
A small gasoline can has a capacity of 0.50 gal.Express this in milliliters.1 gal = 3.785411784 L
A)1.9 × 103 mL
B)1.9 × 10-3 mL
C)1.9 mL
D)1.3 × 102 mL
E)1.3 × 10-4 mL
A)1.9 × 103 mL
B)1.9 × 10-3 mL
C)1.9 mL
D)1.3 × 102 mL
E)1.3 × 10-4 mL

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
Round off each of the following quantities to two significant figures:
i.32,175,000 µm
ii.3.3000 × 107 kg
iii.0.04721 m
iv.8751 cm3
A)i. 3.2 µm ii. 3.3 × 107 kg iii. 0.047 m iv. 8.8 × 103 cm3
B)i. 3.2 × 107 µm ii. 3.3 × 107 kg iii. 0.047 m iv. 8.8 × 103 cm3
C)i. 3.2 × 107 µm ii. 3.3 × 107 kg iii. 0.047 m iv. 8.7 × 103 cm3
D)i. 3.2 × 107 µm ii. 3.3 × 107 kg iii. 0.05 m iv. 8.8 × 103 cm3
E)i. 3.2 × 107 µm ii. 3.3 kg iii. 0.047 m iv. 8.8 × 103 cm3
i.32,175,000 µm
ii.3.3000 × 107 kg
iii.0.04721 m
iv.8751 cm3
A)i. 3.2 µm ii. 3.3 × 107 kg iii. 0.047 m iv. 8.8 × 103 cm3
B)i. 3.2 × 107 µm ii. 3.3 × 107 kg iii. 0.047 m iv. 8.8 × 103 cm3
C)i. 3.2 × 107 µm ii. 3.3 × 107 kg iii. 0.047 m iv. 8.7 × 103 cm3
D)i. 3.2 × 107 µm ii. 3.3 × 107 kg iii. 0.05 m iv. 8.8 × 103 cm3
E)i. 3.2 × 107 µm ii. 3.3 kg iii. 0.047 m iv. 8.8 × 103 cm3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following quantities is/are correctly rounded to three significant figures?
i.0.0277 mL = 0.028 mL
ii.2.1295 km = 2.12 km
iii.3.986 × 104 cm3 = 3.99 × 104 cm3
iv.0.003398216 in = 0.003 in
A)i, ii, and iv
B)ii and iii
C)iii only
D)All are correctly rounded to three significant figures
E)None is correctly rounded to three significant figures
i.0.0277 mL = 0.028 mL
ii.2.1295 km = 2.12 km
iii.3.986 × 104 cm3 = 3.99 × 104 cm3
iv.0.003398216 in = 0.003 in
A)i, ii, and iv
B)ii and iii
C)iii only
D)All are correctly rounded to three significant figures
E)None is correctly rounded to three significant figures

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
What is the volume of 22 g of gold,which has a density of 19.3 g/cm3?
A)21 cm3
B)3 cm3
C)1.1 cm3
D)0.88 cm3
E)4.2 × 102 cm3
A)21 cm3
B)3 cm3
C)1.1 cm3
D)0.88 cm3
E)4.2 × 102 cm3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
What is the volume of a 5.00 g pure aluminum cylinder if its density is 2.70 g/cm3?
A)5.00 cm3
B)1.85 cm3
C)13.5 cm3
D)0.540 cm3
E)7.41 × 10-2 cm3
A)5.00 cm3
B)1.85 cm3
C)13.5 cm3
D)0.540 cm3
E)7.41 × 10-2 cm3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
The energy of a photon of light is directly proportional to the frequency of that photon.Calculate the value of the proportionality constant,given that a photon of energy 4.4 × 10-19 J has a frequency of 6.7 × 1014/s.
A)2.9 × 10-4 J/s
B)2.9 × 10-4 J . s
C)2.9 × 10-4 s/J
D)6.6 × 10-34 J/s
E)6.6 × 10-34 J . s
A)2.9 × 10-4 J/s
B)2.9 × 10-4 J . s
C)2.9 × 10-4 s/J
D)6.6 × 10-34 J/s
E)6.6 × 10-34 J . s

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
Find the mass of 35.7 mL of benzene if its density is 0.877 g/mL.
A)2.46 × 10-2 g
B)3.19 × 10-2 g
C)40.7 g
D)35.7 g
E)31.3 g
A)2.46 × 10-2 g
B)3.19 × 10-2 g
C)40.7 g
D)35.7 g
E)31.3 g

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
Pay is directly proportional to hours worked.What units would be appropriate for the proportionality constant in an equation that relates pay and hours worked?
A)dollars × hours
B)hours × dollars
C)dollars/hour
D)hours/dollar
E)hours2/dollar
A)dollars × hours
B)hours × dollars
C)dollars/hour
D)hours/dollar
E)hours2/dollar

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
Distance and time are directly proportional for an object moving a constant speed.What is the value of the proportionality constant for a car that travels 133 miles in 2.5 hours?
A)3.3 × 102 mi • hr
B)1.4 × 102 mi • hr
C)1.3 × 102 mi • hr
D)53 mi/hr
E)0.019 hr/mi
A)3.3 × 102 mi • hr
B)1.4 × 102 mi • hr
C)1.3 × 102 mi • hr
D)53 mi/hr
E)0.019 hr/mi

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
The defining equation for density (D)is D = 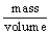 .Which of the following represents units in which density might be expressed?
.Which of the following represents units in which density might be expressed?
A)liters/seconds
B)liters/grams
C)milligrams/miles
D)kilograms/pints
E)density/cubic centimeters
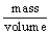 .Which of the following represents units in which density might be expressed?
.Which of the following represents units in which density might be expressed?A)liters/seconds
B)liters/grams
C)milligrams/miles
D)kilograms/pints
E)density/cubic centimeters

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
For a fixed amount of a gas at constant volume,the pressure (P)of the gas is directly proportional to its absolute temperature (T).Which of the following is the best choice for units of the proportionality constant that relates P and T in an equation?
A)atm/K
B)atm/°C
C)K/atm
D)°C/atm
E)°C • atm
A)atm/K
B)atm/°C
C)K/atm
D)°C/atm
E)°C • atm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck


