Deck 16: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 16: Solutions
1
80.0 grams of potassium sulfate is dissolved in 3.20 × 102 grams of water.Find the percentage concentration by mass.
A)15.0%
B)20.0%
C)25.0%
D)30.0%
E)256%
A)15.0%
B)20.0%
C)25.0%
D)30.0%
E)256%
20.0%
2
Calculate the number of grams of ethyl alcohol (ethanol),C2H5OH,in 4.40 × 102 grams of a 23.0% solution.
A)5.23 g
B)19.1 g
C)23.0 g
D)339 g
E)101 g
A)5.23 g
B)19.1 g
C)23.0 g
D)339 g
E)101 g
101 g
3
What volume of 0.372 M H2SO4 contains 0.100 moles of solute?
A)269 mL
B)380 mL
C)372 mL
D)36.0 mL
E)100 mL
A)269 mL
B)380 mL
C)372 mL
D)36.0 mL
E)100 mL
269 mL
4
When a saturated solution is in equilibrium with undissolved solute:
A)the solution separates into layers
B)dissolving and crystallization stop
C)the quantity of dissolved solute equals the quantity of undissolved solute
D)the concentration of the solution remains constant
E)the temperature increases until more solute dissolves
A)the solution separates into layers
B)dissolving and crystallization stop
C)the quantity of dissolved solute equals the quantity of undissolved solute
D)the concentration of the solution remains constant
E)the temperature increases until more solute dissolves

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
The contents of the beaker shown below were the result of thoroughly mixing two liquids and then letting the beaker stand undisturbed.  The two liquids were methanol (CH3OH,d = 0.787 g/mL)and toluene (C6H5CH3 d = 0.866 g/mL).
The two liquids were methanol (CH3OH,d = 0.787 g/mL)and toluene (C6H5CH3 d = 0.866 g/mL).
A)These two liquids are miscible with methanol forming the top layer.
B)These two liquids are immiscible with methanol forming the bottom layer.
C)These two liquids are miscible with toluene forming the top layer.
D)These two liquids are immiscible with toluene forming the bottom layer.
 The two liquids were methanol (CH3OH,d = 0.787 g/mL)and toluene (C6H5CH3 d = 0.866 g/mL).
The two liquids were methanol (CH3OH,d = 0.787 g/mL)and toluene (C6H5CH3 d = 0.866 g/mL).A)These two liquids are miscible with methanol forming the top layer.
B)These two liquids are immiscible with methanol forming the bottom layer.
C)These two liquids are miscible with toluene forming the top layer.
D)These two liquids are immiscible with toluene forming the bottom layer.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the incorrect statement about events that occur between the time excess solid solute is first introduced to a liquid solvent and the time when the solution becomes saturated.Assume that the surface area of the solute remains constant throughout the process.
A)The rate of concentration change is zero when the solution is saturated
B)The net rate of concentration change is greatest at the beginning of the process
C)The rate of dissolving is greater than the rate of crystallization when the solution is one-half saturated
D)If temperature remains constant, the rate of dissolving is constant
E)If temperature remains constant, the rate of crystallization remains constant
A)The rate of concentration change is zero when the solution is saturated
B)The net rate of concentration change is greatest at the beginning of the process
C)The rate of dissolving is greater than the rate of crystallization when the solution is one-half saturated
D)If temperature remains constant, the rate of dissolving is constant
E)If temperature remains constant, the rate of crystallization remains constant

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Stirring a solute into a solution increases the net dissolving rate (reduces the dissolving time to reach equilibrium)because the:
A)dissolving rate remains constant while the crystallization rate is reduced
B)dissolving rate remains constant while the crystallization rate is increased
C)dissolving rate is increased while the crystallization rate is reduced
D)dissolving rate is increased while the crystallization rate remains constant
E)dissolving rate is reduced while the crystallization rate remains constant
A)dissolving rate remains constant while the crystallization rate is reduced
B)dissolving rate remains constant while the crystallization rate is increased
C)dissolving rate is increased while the crystallization rate is reduced
D)dissolving rate is increased while the crystallization rate remains constant
E)dissolving rate is reduced while the crystallization rate remains constant

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following correctly applies to the term "solution"?
A)heterogeneous mixture
B)variable composition
C)constant properties
D)dissolved particles can are usually larger than about 10-7 cm
E)exist only in the liquid and gas state
A)heterogeneous mixture
B)variable composition
C)constant properties
D)dissolved particles can are usually larger than about 10-7 cm
E)exist only in the liquid and gas state

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the following beaker which has had its contents (KBr and water)mixed until no further change occurs.  Which of the following best describes the contents of the beaker?
Which of the following best describes the contents of the beaker?
A)KBr - solute and water - solvent.
B)Saturated solution
C)Addition of enough water would produce an unsaturated solution
D)A heterogeneous mixture.
E)All of the above correctly describe the contents of the beaker..
 Which of the following best describes the contents of the beaker?
Which of the following best describes the contents of the beaker?A)KBr - solute and water - solvent.
B)Saturated solution
C)Addition of enough water would produce an unsaturated solution
D)A heterogeneous mixture.
E)All of the above correctly describe the contents of the beaker..

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
How many grams of Ba(OH)2 are needed to prepare 2.50 liters of 0.200 M barium hydroxide solution?
A)85.7 g
B)77.2 g
C)34.3 g
D)13.7 g
E)171.3 g
A)85.7 g
B)77.2 g
C)34.3 g
D)13.7 g
E)171.3 g

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
If ethane,CH3CH3,a gas,is bubbled into methanol,CH3OH,a liquid,it is logical to expect that this gas:
A)will dissolve in the methanol because of similar intermolecular attractions
B)will dissolve in the methanol because their molar masses are so close
C)will dissolve in the methanol because both molecules are polar
D)will not dissolve in the methanol because one molecule exhibits hydrogen bonding and the other does not
E)will not dissolve in the methanol because their molar masses are so close
A)will dissolve in the methanol because of similar intermolecular attractions
B)will dissolve in the methanol because their molar masses are so close
C)will dissolve in the methanol because both molecules are polar
D)will not dissolve in the methanol because one molecule exhibits hydrogen bonding and the other does not
E)will not dissolve in the methanol because their molar masses are so close

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the graph shown below. 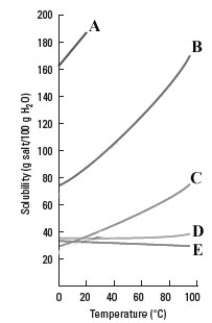 Which substance's solubility behaves the most like the solubility of a gas as the temperature is increased?
Which substance's solubility behaves the most like the solubility of a gas as the temperature is increased?
A)A
B)B
C)C
D)D
E)E
 Which substance's solubility behaves the most like the solubility of a gas as the temperature is increased?
Which substance's solubility behaves the most like the solubility of a gas as the temperature is increased?A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is incorrect?
A)If sugar is dissolved in water, sugar is the solute and water is the solvent
B)In an experiment, students are instructed to add water to a certain quantity of a stock solution of sodium hydroxide. The resulting solution may be called dilute, and the stock solution is said to be concentrated
C)If, at a given temperature, the solubility of a certain compound is 56 grams per 100 milliliters of water, a solution having a concentration of 46 grams per 100 milliliters at the same temperature is supersaturated
D)Oil and water are immiscible
E)The concentration of a saturated solution, expressed in grams per 100 milliliters of solvent, is the same as its solubility expressed in the same units
A)If sugar is dissolved in water, sugar is the solute and water is the solvent
B)In an experiment, students are instructed to add water to a certain quantity of a stock solution of sodium hydroxide. The resulting solution may be called dilute, and the stock solution is said to be concentrated
C)If, at a given temperature, the solubility of a certain compound is 56 grams per 100 milliliters of water, a solution having a concentration of 46 grams per 100 milliliters at the same temperature is supersaturated
D)Oil and water are immiscible
E)The concentration of a saturated solution, expressed in grams per 100 milliliters of solvent, is the same as its solubility expressed in the same units

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
What is the molality of a solution made by dissolving 12.73 g of CH3OH in 87.27 g of water?
A)6.855 m
B)4.552 m
C)3.977 m
D)1.273 m
E)0.1273 m
A)6.855 m
B)4.552 m
C)3.977 m
D)1.273 m
E)0.1273 m

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
The structures of cyclohexane and benzene are shown below. 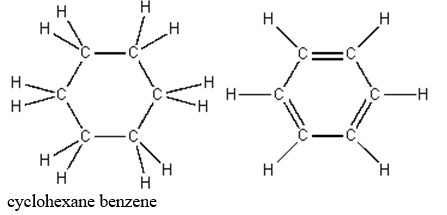
It is logical to expect that these liquids are:
A)miscible because of their similar structures and sizes
B)miscible because they contain the same number of carbon atoms
C)immiscible because benzene, with half as many hydrogen atoms, has less hydrogen bonding than cyclohexane
D)immiscible because one molecule is polar and the other nonpolar
E)immiscible because both molecules have ring structures

It is logical to expect that these liquids are:
A)miscible because of their similar structures and sizes
B)miscible because they contain the same number of carbon atoms
C)immiscible because benzene, with half as many hydrogen atoms, has less hydrogen bonding than cyclohexane
D)immiscible because one molecule is polar and the other nonpolar
E)immiscible because both molecules have ring structures

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the graph shown below. 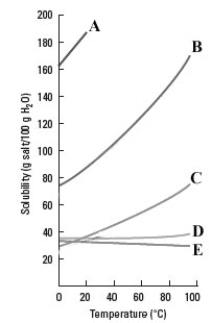 Which substance shows the smallest increase in solubility as the temperature is increased?
Which substance shows the smallest increase in solubility as the temperature is increased?
A)A
B)B
C)C
D)D
E)E
 Which substance shows the smallest increase in solubility as the temperature is increased?
Which substance shows the smallest increase in solubility as the temperature is increased?A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
The cylinder shown contains 0.79 moles of nitrogen,0.19 moles of oxygen and 0.02 moles carbon dioxide,a total of 1.00 mole of molecules in the approximate proportion in which they are present in air.Of the three gases,only carbon dioxide is appreciably soluble in the water in the well at the bottom.Assume an equilibrium between dissolved and undissolved carbon dioxide at the beginning and sufficient time lapse to reestablish that equilibrium after the change described. 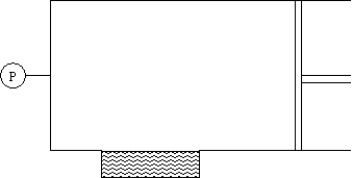 If 0.02 mole of carbon dioxide is forced into the cylinder,the solubility of carbon dioxide:
If 0.02 mole of carbon dioxide is forced into the cylinder,the solubility of carbon dioxide:
A)increases by a factor of about 50
B)increases by a factor of about 2
C)increases by 2%
D)remains unchanged
E)decreases
 If 0.02 mole of carbon dioxide is forced into the cylinder,the solubility of carbon dioxide:
If 0.02 mole of carbon dioxide is forced into the cylinder,the solubility of carbon dioxide:A)increases by a factor of about 50
B)increases by a factor of about 2
C)increases by 2%
D)remains unchanged
E)decreases

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
You are required to prepare a 3.50% AgNO3 solution,using 5.00 g of silver nitrate.What mass of water will be needed?
A)143 g
B)148 g
C)138 g
D)125 g
E)17.5 g
A)143 g
B)148 g
C)138 g
D)125 g
E)17.5 g

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
If 45.5 g of BaCl2 is dissolved in water to produce 2.74 L of solution,what is the molarity of the solution?
A)0.219 M
B)0.599 M
C)0.263 M
D)0.0797 M
E)0.0962 M
A)0.219 M
B)0.599 M
C)0.263 M
D)0.0797 M
E)0.0962 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is incorrect?
A)The roles of solute and solvent are not always clearly defined in the solution of one liquid in another
B)When water and carbon tetrachloride are in the same test tube, a denser layer of carbon tetrachloride forms beneath a less dense layer of water. The liquids are said to be miscible
C)A solution of a given concentration that is saturated at one temperature may be unsaturated at another temperature
D)Dilute nitric acid and dilute sulfuric acid may or may not have the same concentration
E)The concentration of solute in a supersaturated solution is greater than the normal solubility limit
A)The roles of solute and solvent are not always clearly defined in the solution of one liquid in another
B)When water and carbon tetrachloride are in the same test tube, a denser layer of carbon tetrachloride forms beneath a less dense layer of water. The liquids are said to be miscible
C)A solution of a given concentration that is saturated at one temperature may be unsaturated at another temperature
D)Dilute nitric acid and dilute sulfuric acid may or may not have the same concentration
E)The concentration of solute in a supersaturated solution is greater than the normal solubility limit

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
What volume of water,which has a density of 1.00 g/mL,must be added to 25.0 g of CH3CH2OH to form a solution that is 0.100 molal?
A)2.50 L
B)0.250 L
C)5.43 L
D)0.543 L
E)4.00 L
A)2.50 L
B)0.250 L
C)5.43 L
D)0.543 L
E)4.00 L

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
75.0 mL of water is added to 12.9 mL of 0.250 M KMnO4.What is the concentration of the diluted solution?
A)1.70 M
B)1.45 M
C)0.0581 M
D)0.0430 M
E)0.0367 M
A)1.70 M
B)1.45 M
C)0.0581 M
D)0.0430 M
E)0.0367 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
A 50.0 mL hydrochloric acid sample is analyzed for its concentration by titration.Determine the concentration of the sample,given that it requires 21.50 mL of 0.102 M sodium hydroxide solution to neutralize the acid.
A)0.105 M
B)1.05 M
C)22.8 M
D)0.0439 M
E)0.237 M
A)0.105 M
B)1.05 M
C)22.8 M
D)0.0439 M
E)0.237 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
Cu(OH)2 can be obtained from the addition of excess NaOH solution to a solution of Cu(NO3)2 as shown in the figure. 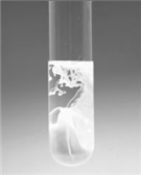 If the NaOH is added to 35.0 mL of 0.167 M Cu(NO3)2 and the precipitate isolated by filtration,what is the theoretical yield of the reaction?
If the NaOH is added to 35.0 mL of 0.167 M Cu(NO3)2 and the precipitate isolated by filtration,what is the theoretical yield of the reaction?
A)0.471 g
B)585 g
C)1.10 g
D)0.570 g
E)1.14 g
 If the NaOH is added to 35.0 mL of 0.167 M Cu(NO3)2 and the precipitate isolated by filtration,what is the theoretical yield of the reaction?
If the NaOH is added to 35.0 mL of 0.167 M Cu(NO3)2 and the precipitate isolated by filtration,what is the theoretical yield of the reaction?A)0.471 g
B)585 g
C)1.10 g
D)0.570 g
E)1.14 g

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
What volume of hydrogen gas,measured at STP,is produced in the reaction of excess aluminum with 50.0 mL of 0.935 M hydrochloric acid?
A)0.531 L
B)1.06 L
C)2.12 L
D)0.0474 L
E)0.0237 L
A)0.531 L
B)1.06 L
C)2.12 L
D)0.0474 L
E)0.0237 L

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the normality of an unknown acid if 21.8 mL is required to titrate a 25.0 mL sample of 1.02 N NaOH.
A)1.02 N
B)1.17 N
C)0.855 N
D)0.889 N
E)There is insufficient information to answer this question
A)1.02 N
B)1.17 N
C)0.855 N
D)0.889 N
E)There is insufficient information to answer this question

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
20.5 milliliters of 0.506 M NaOH is required to titrate 25.0 milliliters of sulfuric acid of unknown concentration.Find the molarity of the acid.
A)0.207 M
B)0.309 M
C)0.415 M
D)0.830 M
E)4.82 M
A)0.207 M
B)0.309 M
C)0.415 M
D)0.830 M
E)4.82 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the normality of a potassium hydroxide solution if 27.7 mL of the solution is needed to neutralize 0.477 g of sulfamic acid in the reaction NH2SO3H + KOH → NH2SO3K + H2O.
A)3.57 × 10-4 N
B)1.77 × 10-4 N
C)4.38 × 103 N
D)5.64 N
E)0.177 N
A)3.57 × 10-4 N
B)1.77 × 10-4 N
C)4.38 × 103 N
D)5.64 N
E)0.177 N

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
What volume of 0.142 N hydrochloric acid will react with 10.00 mL of 0.117 N barium hydroxide?
A)12.14 mL
B)6.07 mL
C)8.24 mL
D)16.48 mL
E)10.00 mL
A)12.14 mL
B)6.07 mL
C)8.24 mL
D)16.48 mL
E)10.00 mL

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
A hydrochloric acid solution is standardized by titrating against solid sodium carbonate as shown in the following image.The HCl is in the buret and the sodium carbonate in the flask.  The equation is 2 HCl(aq)+ Na2CO3(s)→ 2 NaCl(aq)+ H2O(
The equation is 2 HCl(aq)+ Na2CO3(s)→ 2 NaCl(aq)+ H2O( 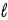 )+ CO2(g).If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
)+ CO2(g).If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
A)0.0316 M
B)0.0633 M
C)7.90 M
D)0.253 M
E)0.127 M
 The equation is 2 HCl(aq)+ Na2CO3(s)→ 2 NaCl(aq)+ H2O(
The equation is 2 HCl(aq)+ Na2CO3(s)→ 2 NaCl(aq)+ H2O( 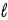 )+ CO2(g).If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
)+ CO2(g).If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?A)0.0316 M
B)0.0633 M
C)7.90 M
D)0.253 M
E)0.127 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
Write the balanced equation for the reaction of aluminum chloride with silver nitrate and determine what volume of 0.107 M silver nitrate is required to precipitate all the chloride from 15.00 mL of 0.0500 M aluminum chloride.
A)45.0 mL
B)7.01 mL
C)21.0 mL
D)63.1 mL
E)15.0 mL
A)45.0 mL
B)7.01 mL
C)21.0 mL
D)63.1 mL
E)15.0 mL

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
The citric acid in a lemon juice sample was neutralized by titration with NaOH solution.If 5.00 mL of lemon juice required 47.8 mL of 0.121 M NaOH for neutralization,what was the molarity of the citric acid in the lemon juice? The reaction is 3 NaOH + H3C6H5O7 → 3 H2O + Na3C6H5O7.
A)1.16 M
B)3.47 M
C)0.110 M
D)0.386 M
E)0.329 M
A)1.16 M
B)3.47 M
C)0.110 M
D)0.386 M
E)0.329 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
Potassium hydrogen phthalate is used as a primary standard in finding the concentration of a solution of sodium hydroxide by the reaction KHC8H4O4 + NaOH → NaKC8H4O4 + H2O.What is the molarity of the base if 32.75 mL is required to titrate 1.732 g of the primary standard?
A)0.5189 M
B)0.2590 M
C)1.732 M
D)3.861 M
E)3.275 M
A)0.5189 M
B)0.2590 M
C)1.732 M
D)3.861 M
E)3.275 M

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the volumetric flask shown below.The volume of the flask is 1.000 L.  Nitric acid is commercially available at a concentration of 15.9 M.What volume of this solution must be diluted to in this flask to prepare a 4.00 M solution?
Nitric acid is commercially available at a concentration of 15.9 M.What volume of this solution must be diluted to in this flask to prepare a 4.00 M solution?
A)400 mL
B)39.8 mL
C)398 mL
D)25.2 mL
E)252 mL
 Nitric acid is commercially available at a concentration of 15.9 M.What volume of this solution must be diluted to in this flask to prepare a 4.00 M solution?
Nitric acid is commercially available at a concentration of 15.9 M.What volume of this solution must be diluted to in this flask to prepare a 4.00 M solution?A)400 mL
B)39.8 mL
C)398 mL
D)25.2 mL
E)252 mL

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
1.25 g C6H5COOH is dissolved in water and the solution is titrated with 33.5 mL of a solution of NaOH of an unknown concentration.Calculate the normality of the NaOH for the reaction C6H5COOH(aq)+ NaOH(aq)→ C6H5COONa(aq)+ H2O( 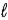 ).
).
A)3.06 × 10-4 N
B)3.43 × 10-4 N
C)0.306 N
D)3.27 N
E)4.55 × 103 N
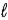 ).
).A)3.06 × 10-4 N
B)3.43 × 10-4 N
C)0.306 N
D)3.27 N
E)4.55 × 103 N

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
What mass of cobalt(II)phosphate will precipitate when excess sodium phosphate solution is added to 100.0 mL 0.500 M cobalt(II)nitrate?
A)6.11 g
B)9.2 g
C)18.3 g
D)36.7 g
E)55.0 g
A)6.11 g
B)9.2 g
C)18.3 g
D)36.7 g
E)55.0 g

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
What is the equivalent mass of sulfuric acid in the reaction H2SO4 + 2 NaOH → 2 H2O + Na2SO4?
A)98.0 g/eq
B)1.00 g/eq
C)2.00 g/eq
D)49.0 g/eq
E)9.80 g/eq
A)98.0 g/eq
B)1.00 g/eq
C)2.00 g/eq
D)49.0 g/eq
E)9.80 g/eq

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the normality of a solution made by dissolving 10.0 g of KOH in water to produce 125 mL of solution.
A)1.43 N
B)0.178 N
C)0.713 N
D)2.86 N
E)1.25 N
A)1.43 N
B)0.178 N
C)0.713 N
D)2.86 N
E)1.25 N

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
Dilute laboratory bench reagents are generally 6.0 M.What volume of dilute HCl must be used to prepare 5.00 × 102 mL of 0.25 M HCl?
A)8.3 mL
B)21 mL
C)12 mL
D)50 mL
E)42 mL
A)8.3 mL
B)21 mL
C)12 mL
D)50 mL
E)42 mL

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
How many equivalents of sulfuric acid will react with 0.15 eq of barium hydroxide?
A)0.075 eq
B)0.30 eq
C)0.45 eq
D)0.60 eq
E)0.15 eq
A)0.075 eq
B)0.30 eq
C)0.45 eq
D)0.60 eq
E)0.15 eq

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
A pure solvent freezes at 43.4°C.The freezing temperature is 40.7°C when 17.5 g of CO(NH2)2 is dissolved in 1.00 × 102 g of the solvent.Calculate the molal freezing point constant of the solvent.
A)0.027 °C/m
B)0.343 °C/m
C)0.93 °C/m
D)13.9 °C/m
E)14.9 °C/m
A)0.027 °C/m
B)0.343 °C/m
C)0.93 °C/m
D)13.9 °C/m
E)14.9 °C/m

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
When 2.77 g of a solute was dissolved in 25.0 g of water,the resulting solution had a freezing point of -3.06°C.What is the apparent molar mass of the solute? Kf of water is 1.86°C/m.
A)36.2 g/mol
B)67.3 g/mol
C)227 g/mol
D)82.2 g/mol
E)1.65 g/mol
A)36.2 g/mol
B)67.3 g/mol
C)227 g/mol
D)82.2 g/mol
E)1.65 g/mol

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
What is the molal boiling point constant for a solvent that has a boiling point elevation of 2.5°C when 10.0 g of a solute of molar mass 72.0 g/mol is dissolved in 0.314 kg of the solvent?
A)5.7 °C/m
B)18 °C/m
C)0.25 °C/m
D)0.035 °C/m
E)8.0 °C/m
A)5.7 °C/m
B)18 °C/m
C)0.25 °C/m
D)0.035 °C/m
E)8.0 °C/m

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the decrease in the freezing point of a solution prepared by dissolving 5.02 grams of paradichlorobenzene,C6H4Cl2,in 75.0 grams of naphthalene,C10H8.Kf for naphthalene is 6.9°C/m.
A)0.24°C
B)0.46°C
C)3.1°C
D)15°C
E)35°C
A)0.24°C
B)0.46°C
C)3.1°C
D)15°C
E)35°C

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the increase in boiling point of a solution made by dissolving 40.0 g of glucose,C6H12O6,in 250.0 g of water.Kb for water is 0.52°C/m.
A)0.12°C
B)0.46°C
C)0.59°C
D)1.7°C
E)83.2°C
A)0.12°C
B)0.46°C
C)0.59°C
D)1.7°C
E)83.2°C

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
Silver nitrate is commonly used to determine the chloride ion concentration in an aqueous solution.37.0 mL of 0.174 N AgNO3 is required to titrate the chloride ion in a 20.0 mL sample of zinc chloride solution.What is the normality of the zinc chloride? 2 AgNO3(aq)+ ZnCl2(aq)→ 2 AgCl(s)+ Zn(NO3)2(aq)
A)0.0470 N
B)0.0941 N
C)0.161 N
D)0.322 N
E)0.644 N
A)0.0470 N
B)0.0941 N
C)0.161 N
D)0.322 N
E)0.644 N

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
An unknown substance is known to be a nonelectrolyte,and it dissolves in water.Determine the approximate molar mass of the substance if 37.7 g of the substance dissolved in 1.00 × 102 mL of water has a boiling temperature of 102.73°C.Kb for water is 0.52°C/m.
A)5.3 g/mol
B)1.4 g/mol
C)2.0 × 102 g/mol
D)3.8 × 102 g/mol
E)72 g/mol
A)5.3 g/mol
B)1.4 g/mol
C)2.0 × 102 g/mol
D)3.8 × 102 g/mol
E)72 g/mol

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck


