Deck 15: Gases, Liquids, and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 15: Gases, Liquids, and Solids
1
Acetone,a highly volatile liquid,is placed in a closed container.The amount of liquid decreases,but eventually becomes stable.The volume of the closed space above the liquid is increased.The amount of liquid then...
A)May or may not change, but no prediction can be made without additional information about temperature
B)May or may not change, but no prediction can be made without additional information about the magnitude of the volume increase
C)Remains the same
D)Increases
E)Decreases
A)May or may not change, but no prediction can be made without additional information about temperature
B)May or may not change, but no prediction can be made without additional information about the magnitude of the volume increase
C)Remains the same
D)Increases
E)Decreases
Decreases
2
The total pressure exerted by the oxygen saturated with water vapor is 764 torr at a temperature at which water vapor pressure is 26 torr.Find the partial pressure of the oxygen.
A)0.0340 torr
B)29.4 torr
C)738 torr
D)790 torr
E)1.99 × 104 torr
A)0.0340 torr
B)29.4 torr
C)738 torr
D)790 torr
E)1.99 × 104 torr
738 torr
3
Predict the order in which boiling points of the following hydrides decrease (highest boiling point first,lowest last): PH3,AsH3,SbH3 (As is atomic number 33; Sb is atomic number 51).
A)PH3, AsH3, SbH3
B)AsH3, SbH3, PH3
C)SbH3, AsH3, PH3
D)PH3, SbH3, AsH3
E)SbH3, PH3, AsH3
A)PH3, AsH3, SbH3
B)AsH3, SbH3, PH3
C)SbH3, AsH3, PH3
D)PH3, SbH3, AsH3
E)SbH3, PH3, AsH3
SbH3, AsH3, PH3
4
Identify the most correct statement comparing intermolecular attractions (IMA)in the liquid and gaseous states of the same substance:
A)IMA are weaker in the gaseous state because the molecules are larger at the higher temperature
B)IMA are weaker in the gaseous state because the molecules are hotter
C)IMA are weaker in the gaseous state because molecules move at slower speeds
D)IMA are weaker in the gaseous state because molecules are so far apart
E)IMA are weaker in the gaseous state because the molecules fill their container
A)IMA are weaker in the gaseous state because the molecules are larger at the higher temperature
B)IMA are weaker in the gaseous state because the molecules are hotter
C)IMA are weaker in the gaseous state because molecules move at slower speeds
D)IMA are weaker in the gaseous state because molecules are so far apart
E)IMA are weaker in the gaseous state because the molecules fill their container

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
Hydrogen gas can be collected by water displacement when magnesium reacts with hydrochloric acid.When the container is filled with the hydrogen gas,which of the following is true of the total pressure (P)inside the container?
A)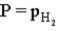
B)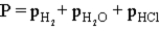
C)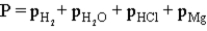
D)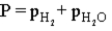
E)P = atmospheric pressure
A)
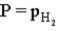
B)
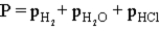
C)
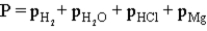
D)
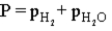
E)P = atmospheric pressure

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is correct?
A)Intermolecular forces are weaker in liquids than in gases because the particles are closer to each other
B)Smaller intermolecular distances in liquids result in stronger intermolecular forces, when compared to intermolecular distances and forces in gases
C)Compared to liquids, large distances between gaseous molecules yield large intermolecular attractions
D)The smaller the distance between molecules the smaller the intermolecular attractions, therefore attractions are weaker in liquids than gases
E)All statements are correct
A)Intermolecular forces are weaker in liquids than in gases because the particles are closer to each other
B)Smaller intermolecular distances in liquids result in stronger intermolecular forces, when compared to intermolecular distances and forces in gases
C)Compared to liquids, large distances between gaseous molecules yield large intermolecular attractions
D)The smaller the distance between molecules the smaller the intermolecular attractions, therefore attractions are weaker in liquids than gases
E)All statements are correct

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
Acetone,a highly volatile liquid,is placed in an open container.The liquid disappears.The vapor pressure of the acetone in the open container...
A)is higher than the equilibrium vapor pressure
B)is lower than the equilibrium vapor pressure
C)is the same as the equilibrium vapor pressure
D)is equal to the vapor pressure of acetone in a closed container
E)cannot be compared to the equilibrium vapor pressure without more information
A)is higher than the equilibrium vapor pressure
B)is lower than the equilibrium vapor pressure
C)is the same as the equilibrium vapor pressure
D)is equal to the vapor pressure of acetone in a closed container
E)cannot be compared to the equilibrium vapor pressure without more information

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
In a gas mixture the partial pressure of helium is 225 torr,of neon,375 torr,and of argon,5 torr.What is the total pressure exerted by the mixture?
A)605 torr
B)4.22 × 105 torr
C)It cannot be calculated without knowledge of container volume
D)It cannot be calculated without atmospheric pressure
E)It cannot be calculated without both container volume and atmospheric pressure
A)605 torr
B)4.22 × 105 torr
C)It cannot be calculated without knowledge of container volume
D)It cannot be calculated without atmospheric pressure
E)It cannot be calculated without both container volume and atmospheric pressure

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following properties of liquids is the least affected by the strength of intermolecular forces?
A)vapor pressure
B)boiling point
C)heat of vaporization
D)density
E)viscosity
A)vapor pressure
B)boiling point
C)heat of vaporization
D)density
E)viscosity

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
With all other factors being equal,which of the following correctly lists intermolecular forces in order of increasing strength?
A)Dipole forces < Induced dipole forces < Hydrogen bonds
B)Induced dipole forces < Dipole forces < Hydrogen bonds
C)Hydrogen bonds < Dipole forces < Induced dipole forces
D)Dipole forces < Hydrogen bonds < Induced dipole forces
E)Induced dipole forces < Hydrogen bonds < Dipole forces
A)Dipole forces < Induced dipole forces < Hydrogen bonds
B)Induced dipole forces < Dipole forces < Hydrogen bonds
C)Hydrogen bonds < Dipole forces < Induced dipole forces
D)Dipole forces < Hydrogen bonds < Induced dipole forces
E)Induced dipole forces < Hydrogen bonds < Dipole forces

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Examine the structure shown below.  Which of the following is the most important intermolecular forces experienced between this molecule and an identical molecule?
Which of the following is the most important intermolecular forces experienced between this molecule and an identical molecule?
A)hydrogen bonds
B)dipole forces
C)induced dipole forces
D)a and c are about the same in this case.
E)b and c are about the same in this case.
 Which of the following is the most important intermolecular forces experienced between this molecule and an identical molecule?
Which of the following is the most important intermolecular forces experienced between this molecule and an identical molecule?A)hydrogen bonds
B)dipole forces
C)induced dipole forces
D)a and c are about the same in this case.
E)b and c are about the same in this case.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
Compared to a substance with induced dipole forces,a substance with hydrogen bond generally has:
A)a higher normal boiling point and vapor pressure, but a lower heat of vaporization
B)a lower normal boiling point, but higher vapor pressure and heat of vaporization
C)higher heat of vaporization but lower normal boiling point and vapor pressure
D)a lower normal boiling point and heat of vaporization, but higher vapor pressure
E)lower vapor pressure, but higher normal boiling point and heat of vaporization
A)a higher normal boiling point and vapor pressure, but a lower heat of vaporization
B)a lower normal boiling point, but higher vapor pressure and heat of vaporization
C)higher heat of vaporization but lower normal boiling point and vapor pressure
D)a lower normal boiling point and heat of vaporization, but higher vapor pressure
E)lower vapor pressure, but higher normal boiling point and heat of vaporization

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the open-ended manometer shown below.In this apparatus the arm on the right is open to the atmosphere and feels the full effect of atmospheric pressure.The pressure in the chemistry lab at the time of this experiment is 755 mm Hg.The flask contains O2,N2 and Ne. 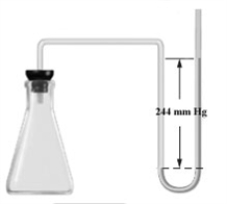 Which of the following correctly applies to this apparatus?
Which of the following correctly applies to this apparatus?
A)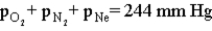
B)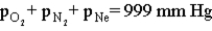
C)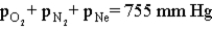
D)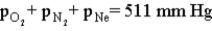
E)none of the above
 Which of the following correctly applies to this apparatus?
Which of the following correctly applies to this apparatus?A)
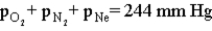
B)
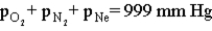
C)
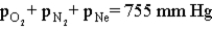
D)
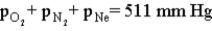
E)none of the above

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
Select from the following the statement that is incorrect or contrary to the generalizations developed in the textbook.
A)The strength of induced dipole forces depends on the ease with which electron distributions can be polarized
B)Dipole forces are present in NF3, but not in CF4
C)The principal intermolecular forces in a straight chain hydrocarbon such as C8H18 are induced dipole forces
D)Intermolecular forces are usually stronger for substances that exhibit hydrogen bonding than for otherwise similar substances lacking hydrogen bonds
E)Induced dipole forces exist in both polar and nonpolar molecular substances, but they are essentially the only attractions in polar compounds
A)The strength of induced dipole forces depends on the ease with which electron distributions can be polarized
B)Dipole forces are present in NF3, but not in CF4
C)The principal intermolecular forces in a straight chain hydrocarbon such as C8H18 are induced dipole forces
D)Intermolecular forces are usually stronger for substances that exhibit hydrogen bonding than for otherwise similar substances lacking hydrogen bonds
E)Induced dipole forces exist in both polar and nonpolar molecular substances, but they are essentially the only attractions in polar compounds

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Draw the Lewis diagrams of CH3OH and water,then from the Lewis diagrams,identify the statement among the following that is most apt to be incorrect.
A)Hydrogen bonds are probably the principal intermolecular force in both compounds
B)Both compounds have both hydrogen bonding and induced dipole forces
C)Both molecules being polar, dipole-dipole forces are present in both compounds
D)Induced dipole forces are present in CH3OH, but not water
E)Induced dipole forces are probably the least significant intermolecular forces in both compounds
A)Hydrogen bonds are probably the principal intermolecular force in both compounds
B)Both compounds have both hydrogen bonding and induced dipole forces
C)Both molecules being polar, dipole-dipole forces are present in both compounds
D)Induced dipole forces are present in CH3OH, but not water
E)Induced dipole forces are probably the least significant intermolecular forces in both compounds

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
Considering the molecular mass and polarity influences on physical properties,which of the following predictions about the boiling points of elemental bromine and chlorine fluoride is most apt to be correct?
A)Both molecular mass and polarity predicts Br2 has the lower boiling point
B)Both molecular mass and polarity predicts Br2 has the higher boiling point
C)Molecular mass predicts Br2 has the lower boiling point, but molecular polarity predicts it has the lower boiling point
D)Molecular mass predicts Br2 has the higher boiling point, but molecular polarity predicts it has the lower boiling point
E)Molecular mass and polarity have an insignificant influence on the physical properties of these molecules
A)Both molecular mass and polarity predicts Br2 has the lower boiling point
B)Both molecular mass and polarity predicts Br2 has the higher boiling point
C)Molecular mass predicts Br2 has the lower boiling point, but molecular polarity predicts it has the lower boiling point
D)Molecular mass predicts Br2 has the higher boiling point, but molecular polarity predicts it has the lower boiling point
E)Molecular mass and polarity have an insignificant influence on the physical properties of these molecules

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
Acetone,a highly volatile liquid,is placed in a closed container.The amount of liquid decreases,but eventually becomes stable.At that time...
A)the rate of evaporation is equal to the rate of condensation
B)the rate of evaporation is less than the rate of condensation
C)the rate of evaporation is greater than the rate of condensation
D)the rates of evaporation and condensation cannot be compared without more information
E)the kinetic energy of each gaseous molecule is equal to the kinetic energy of each liquid molecule
A)the rate of evaporation is equal to the rate of condensation
B)the rate of evaporation is less than the rate of condensation
C)the rate of evaporation is greater than the rate of condensation
D)the rates of evaporation and condensation cannot be compared without more information
E)the kinetic energy of each gaseous molecule is equal to the kinetic energy of each liquid molecule

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
If substance X has stronger intermolecular attractions than substance Y,which of the following is most apt to be false?
A)Y has the lower boiling point and higher vapor pressure
B)Y has the higher vapor pressure and lower molar heat of vaporization
C)Y has the higher boiling point and higher molar heat of vaporization
D)X has the higher boiling point and higher molar heat of vaporization
E)X has the lower vapor pressure and higher molar heat of vaporization
A)Y has the lower boiling point and higher vapor pressure
B)Y has the higher vapor pressure and lower molar heat of vaporization
C)Y has the higher boiling point and higher molar heat of vaporization
D)X has the higher boiling point and higher molar heat of vaporization
E)X has the lower vapor pressure and higher molar heat of vaporization

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
The structural formula for glycerin is shown below.Compare the intermolecular forces in glycerin with those in n-hexane,C6H14,in which all carbons are in a continuous chain.Which of the following statements is true? 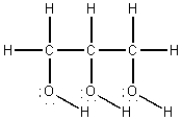
A)The principal intermolecular forces in glycerin are induced dipole forces; in hexane, hydrogen bonds
B)Hydrogen bonding is present in glycerin, but not in hexane
C)Hydrogen bonding is present in both compounds
D)Both compounds exhibit dipole-dipole forces
E)Induced dipole forces are present in neither compound

A)The principal intermolecular forces in glycerin are induced dipole forces; in hexane, hydrogen bonds
B)Hydrogen bonding is present in glycerin, but not in hexane
C)Hydrogen bonding is present in both compounds
D)Both compounds exhibit dipole-dipole forces
E)Induced dipole forces are present in neither compound

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
The kinetic molecular theory as applied to liquids differs in two major ways from the theory as applied to gases.One of these is that:
A)Matter no longer appears to be made up of discrete particles
B)Energy changes result from intermolecular collisions
C)Attractive forces between molecules are significant
D)Molecules are no longer in motion relative to each other
E)Forces between molecules are no longer electrostatic in character
A)Matter no longer appears to be made up of discrete particles
B)Energy changes result from intermolecular collisions
C)Attractive forces between molecules are significant
D)Molecules are no longer in motion relative to each other
E)Forces between molecules are no longer electrostatic in character

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
A sample of solid silver releases 476 J of heat when cooled from 98.3°C to 21.2°C.The specific heat of silver is 0.24 J/g • °C.What is the mass of the sample?
A)8.8 × 103 g
B)1.5 × 105 g
C)0.039 g
D)1.5 g
E)25.7 g
A)8.8 × 103 g
B)1.5 × 105 g
C)0.039 g
D)1.5 g
E)25.7 g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the heat of fusion of an unknown pure substance if 5.33 × 103 kJ of heat is required to melt a 17.2 kg sample.
A)1.09 × 10-5 J/g
B)3.23 × 10-3 J/g
C)3.10 × 102 J/g
D)9.17 × 104 J/g
E)3.10 × 105 J/g
A)1.09 × 10-5 J/g
B)3.23 × 10-3 J/g
C)3.10 × 102 J/g
D)9.17 × 104 J/g
E)3.10 × 105 J/g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the following image. 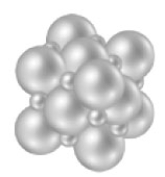 What type of solid is represented by this particulate-level model?
What type of solid is represented by this particulate-level model?
A)amorphous solid
B)molecular crystal
C)ionic crystal
D)covalent network solid
E)metallic crystal
 What type of solid is represented by this particulate-level model?
What type of solid is represented by this particulate-level model?A)amorphous solid
B)molecular crystal
C)ionic crystal
D)covalent network solid
E)metallic crystal

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
Among the following,identify the correct statement about a boiling liquid:
A)The temperature of a liquid boiling in an open beaker is equal to the temperature of the gas space above the liquid
B)Equilibrium vapor pressure at the boiling temperature is higher than the surrounding pressure
C)Vapor pressure in a bubble in the liquid is equal to or slightly greater than the equilibrium pressure at boiling temperature
D)Boiling temperature can be increased by boiling in a vacuum
E)All statements are incorrect
A)The temperature of a liquid boiling in an open beaker is equal to the temperature of the gas space above the liquid
B)Equilibrium vapor pressure at the boiling temperature is higher than the surrounding pressure
C)Vapor pressure in a bubble in the liquid is equal to or slightly greater than the equilibrium pressure at boiling temperature
D)Boiling temperature can be increased by boiling in a vacuum
E)All statements are incorrect

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
A substance that melts at 122°C is probably:
A)A network solid
B)An ionic crystal
C)A metallic crystal
D)A molecular crystal
E)A good electrical conductor
A)A network solid
B)An ionic crystal
C)A metallic crystal
D)A molecular crystal
E)A good electrical conductor

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
The main reason equilibrium vapor pressure is greater at higher temperatures is that...
A)A larger fraction of the molecules in the sample has sufficient kinetic energy to move from the liquid phase to the vapor phase
B)The average kinetic energy is greater
C)Intermolecular attractions are weaker
D)The particles are moving at higher speeds
E)The escape energy is decreased
A)A larger fraction of the molecules in the sample has sufficient kinetic energy to move from the liquid phase to the vapor phase
B)The average kinetic energy is greater
C)Intermolecular attractions are weaker
D)The particles are moving at higher speeds
E)The escape energy is decreased

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
The heat of fusion of gold is 63 joules per gram.Once a 25.0 g sample of gold has been heated to its melting point,1063°C,how much additional heat is required to melt it?
A)89 kJ
B)39 kJ
C)2.7 kJ
D)2.6 kJ
E)1.6 kJ
A)89 kJ
B)39 kJ
C)2.7 kJ
D)2.6 kJ
E)1.6 kJ

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
The specific heat of solid gold is 0.13 J/g • °C.How much heat is needed to raise the temperature of 25.0 g of gold from 23°C to its melting point at 1063°C?
A)3.5 kJ
B)3.4 kJ
C)0.14 kJ
D)2.0 × 103 kJ
E)3.3 kJ
A)3.5 kJ
B)3.4 kJ
C)0.14 kJ
D)2.0 × 103 kJ
E)3.3 kJ

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Why does ice float on liquid water?
A)It is a compound without carbon atoms that exists in the liquid state at room temperature and pressure.
B)The linear structure of the water molecule allows ice molecules to pack together tightly enough to allow ice to float.
C)Ice is structurally similar to wood, so ice floats on water in a manner similar to wooden ships where air becomes trapped in cells within the solid structure.
D)Oxygen's low electronegativity leads to the collapse of the crystal structure, allowing the molecules to become closer together in water.
E)Water molecules in the solid form are held in a crystal pattern that has voids between the molecules.
A)It is a compound without carbon atoms that exists in the liquid state at room temperature and pressure.
B)The linear structure of the water molecule allows ice molecules to pack together tightly enough to allow ice to float.
C)Ice is structurally similar to wood, so ice floats on water in a manner similar to wooden ships where air becomes trapped in cells within the solid structure.
D)Oxygen's low electronegativity leads to the collapse of the crystal structure, allowing the molecules to become closer together in water.
E)Water molecules in the solid form are held in a crystal pattern that has voids between the molecules.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements is correct?
A)There is long-range order in a crystalline solid
B)Graphite is an example of an amorphous solid.
C)Glass, rubber, and plastic are examples of crystalline solids
D)Particles in an amorphous solids are arranged in a distinct geometric order
E)In a crystalline solid particles can move past the closest neighboring particles.
A)There is long-range order in a crystalline solid
B)Graphite is an example of an amorphous solid.
C)Glass, rubber, and plastic are examples of crystalline solids
D)Particles in an amorphous solids are arranged in a distinct geometric order
E)In a crystalline solid particles can move past the closest neighboring particles.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
How are network solids,such as diamond,C,and quartz,SiO2,distinguished from molecular crystals such as I2?
A)Network solids have very high melting points
B)Network solids are ionic, while molecular ones are covalent substances
C)Network solids dissolve in water; molecular ones do not
D)Network solids are excellent conductors of electricity, while molecular crystals are nonconductors
E)Network solids have individual molecules of fixed size; molecular crystals do not
A)Network solids have very high melting points
B)Network solids are ionic, while molecular ones are covalent substances
C)Network solids dissolve in water; molecular ones do not
D)Network solids are excellent conductors of electricity, while molecular crystals are nonconductors
E)Network solids have individual molecules of fixed size; molecular crystals do not

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements about change of state is false?
A)The boiling point of a liquid depends on the gas pressure above the liquid
B)Condensation is an endothermic process
C)Evaporation occurs at the surface of a liquid, but boiling may occur at any point within the liquid
D)Energy is absorbed by a liquid as it boils
E)A liquid boils when its vapor pressure is slightly greater than the pressure above it
A)The boiling point of a liquid depends on the gas pressure above the liquid
B)Condensation is an endothermic process
C)Evaporation occurs at the surface of a liquid, but boiling may occur at any point within the liquid
D)Energy is absorbed by a liquid as it boils
E)A liquid boils when its vapor pressure is slightly greater than the pressure above it

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
The graph below represents a temperature versus energy plot for a pure substance. 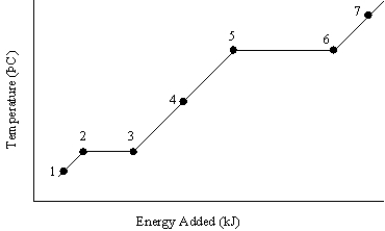 Identify the point(s)where:
Identify the point(s)where:
(i)only gas exists
(ii)both solid and liquid exist
A)(i) 7 (ii) 2 and 3
B)(i) 7 (ii) 5 and 6
C)(i) 1 (ii) 2 and 3
D)(i) 4 (ii) 5 and 6
E)(i) 4 (ii) 2 and 3
 Identify the point(s)where:
Identify the point(s)where:(i)only gas exists
(ii)both solid and liquid exist
A)(i) 7 (ii) 2 and 3
B)(i) 7 (ii) 5 and 6
C)(i) 1 (ii) 2 and 3
D)(i) 4 (ii) 5 and 6
E)(i) 4 (ii) 2 and 3

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
A sample of lead releases 221 kJ of heat when freezing.What is the mass of the sample?
 Hfus = 23 J/g
Hfus = 23 J/g
A)9.6 g
B)9.6 × 103 g
C)5.1 × 106 g
D)2.0 × 10-7 g
E)1.0 × 10-4 g
 Hfus = 23 J/g
Hfus = 23 J/gA)9.6 g
B)9.6 × 103 g
C)5.1 × 106 g
D)2.0 × 10-7 g
E)1.0 × 10-4 g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
Among the following,identify the incorrect statement about a boiling liquid:
A)Boiling temperature may be increased by raising surrounding pressure
B)The temperature of the liquid must be greater than that of the gas above the liquid
C)Boiling in an open beaker occurs at a lower temperature at higher altitudes
D)Equilibrium vapor pressure at boiling temperature equals, or is slightly higher than surrounding pressure
E)All statements are correct
A)Boiling temperature may be increased by raising surrounding pressure
B)The temperature of the liquid must be greater than that of the gas above the liquid
C)Boiling in an open beaker occurs at a lower temperature at higher altitudes
D)Equilibrium vapor pressure at boiling temperature equals, or is slightly higher than surrounding pressure
E)All statements are correct

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
What quantity of water will form by the condensation of steam when 276 kJ of heat are removed?
 Hvap = 2.26 kJ/g
Hvap = 2.26 kJ/g
A)122 g
B)67.8 g
C)2.65 g
D)624 g
E)18.0 g
 Hvap = 2.26 kJ/g
Hvap = 2.26 kJ/gA)122 g
B)67.8 g
C)2.65 g
D)624 g
E)18.0 g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the specific heat of an unknown metal if a 123 gram sample requires 8.8 kJ of heat to change from 16°C to 97°C.
A)0.88 J/g • °C
B)1.1 J/g • °C
C)5.8 × 103 J/g • °C
D)1.3 × 104 J/g • °C
E)8.8 × 107 J/g • °C
A)0.88 J/g • °C
B)1.1 J/g • °C
C)5.8 × 103 J/g • °C
D)1.3 × 104 J/g • °C
E)8.8 × 107 J/g • °C

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
Closed system A consists of liquid acetone in equilibrium with its own vapor at 30°C.System B is like System A,except that the volume of liquid,the area of the liquid surface,and the volume of the vapor space above the liquid are all twice as large as System A,and the temperature is only 15°C.Identify the statement among the following that is positively false.
A)Evaporation and condensation rates are equal in A
B)Evaporation and condensation rates are equal in B
C)Evaporation rate in A is greater than evaporation rate in B
D)Vapor pressure in B is greater than vapor pressure in A
E)Condensation rate in A is greater than condensation rate in B
A)Evaporation and condensation rates are equal in A
B)Evaporation and condensation rates are equal in B
C)Evaporation rate in A is greater than evaporation rate in B
D)Vapor pressure in B is greater than vapor pressure in A
E)Condensation rate in A is greater than condensation rate in B

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the heat of vaporization of zinc if 393 kJ is required to vaporize a 223-gram sample.
A)1.14 × 10-5 kJ/g
B)8.76 × 104 kJ/g
C)0.567 kJ/g
D)1.76 kJ/g
E)2.26 kJ/g
A)1.14 × 10-5 kJ/g
B)8.76 × 104 kJ/g
C)0.567 kJ/g
D)1.76 kJ/g
E)2.26 kJ/g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
Among the following,identify the sentence that states a fact about equilibrium vapor pressure,followed by an acceptable explanation or reason for the fact.
A)Vapor pressure decreases with temperature because the liquid expands and molecules are farther apart
B)Vapor pressure increases with temperature because intermolecular attractions are weaker at higher temperatures
C)Vapor pressure decreases with temperature because molecules stick together better when they are hot
D)Vapor pressure increases with temperature because energy is distributed more efficiently at higher temperatures
E)Vapor pressure increases with temperature because at higher temperatures a larger fraction of the molecules has sufficient energy to escape from the liquid state
A)Vapor pressure decreases with temperature because the liquid expands and molecules are farther apart
B)Vapor pressure increases with temperature because intermolecular attractions are weaker at higher temperatures
C)Vapor pressure decreases with temperature because molecules stick together better when they are hot
D)Vapor pressure increases with temperature because energy is distributed more efficiently at higher temperatures
E)Vapor pressure increases with temperature because at higher temperatures a larger fraction of the molecules has sufficient energy to escape from the liquid state

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
The graph below represents a temperature versus energy plot for a pure substance.Identify the point(s)...(i)where only liquid exists and (ii)is associated with the heat of vaporization 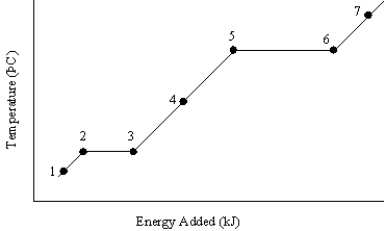
A)(i) 7 (ii) 2 and 3
B)(i) 7 (ii) 5 and 6
C)(i) 4 (ii) 2 and 3
D)(i) 4 (ii) 5 and 6
E)(i) 1 (ii) 5 and 6

A)(i) 7 (ii) 2 and 3
B)(i) 7 (ii) 5 and 6
C)(i) 4 (ii) 2 and 3
D)(i) 4 (ii) 5 and 6
E)(i) 1 (ii) 5 and 6

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
What is the total heat flow if 18 grams of water at 12°C is heated to become steam at 109°C? The specific heat of liquid water is 4.18 J/g • 0°C; the specific heat of steam is 2.0 J/g • 0°C.The heat of vaporization of water is 2.26 kJ/g,and the boiling point of water is 100°C.
A)0.32 kJ
B)6.6 kJ
C)48 kJ
D)41 kJ
E)7.0 × 103 kJ
A)0.32 kJ
B)6.6 kJ
C)48 kJ
D)41 kJ
E)7.0 × 103 kJ

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
The graph below represents a temperature versus energy plot for a pure substance. 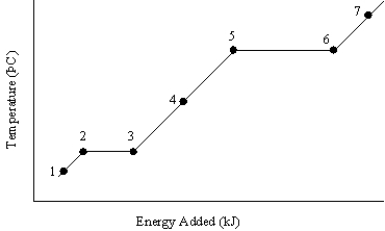 Identify the point(s) (i)where both liquid and gas exist and (ii)is associated with the heat of condensation
Identify the point(s) (i)where both liquid and gas exist and (ii)is associated with the heat of condensation
A)(i) 1 (ii) 5 and 6
B)(i) 2 and 3 (ii) 5 and 6
C)(i) 4 (ii) 5 and 6
D)(i) 5 and 6 (ii) 5 and 6
E)(i) 7 (ii) 3 and 3
 Identify the point(s) (i)where both liquid and gas exist and (ii)is associated with the heat of condensation
Identify the point(s) (i)where both liquid and gas exist and (ii)is associated with the heat of condensationA)(i) 1 (ii) 5 and 6
B)(i) 2 and 3 (ii) 5 and 6
C)(i) 4 (ii) 5 and 6
D)(i) 5 and 6 (ii) 5 and 6
E)(i) 7 (ii) 3 and 3

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
What is the total heat flow if 27 grams of steam at 143°C is cooled to become water at 34°C? The specific heat of water is 4.18 J/g • °C; the specific heat of steam is 2.0 J/g • °C.The heat of vaporization of water is 2.26 kJ/g,and the boiling point of water is 100°C.
A)2.3 kJ
B)7.4 kJ
C)61 kJ
D)71 kJ
E)9.8 × 103 kJ
A)2.3 kJ
B)7.4 kJ
C)61 kJ
D)71 kJ
E)9.8 × 103 kJ

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
What is the total heat flow if 28 grams of water at 12°C is cooled to become ice at -19°C? The specific heat of liquid water is 4.18 J/g • °C; the specific heat of ice is 2.1 J/g • °C.The heat of fusion of ice is 333 J/g,and the freezing point of water is 0.0°C.
A)1.1 kJ
B)1.4 kJ
C)9.4 kJ
D)10.8 kJ
E)11.8 kJ
A)1.1 kJ
B)1.4 kJ
C)9.4 kJ
D)10.8 kJ
E)11.8 kJ

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


