Deck 6: Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 6: Thermochemistry
1
Which of the following is true if ΔEsys = -95 J?
A)The system is gaining 95 J,while the surroundings are losing 95 J.
B)The system is losing 95 J,while the surroundings are gaining 95 J.
C)Both the system and the surroundings are gaining 95 J.
D)Both the system and the surroundings are losing 95 J.
E)None of the above are true.
A)The system is gaining 95 J,while the surroundings are losing 95 J.
B)The system is losing 95 J,while the surroundings are gaining 95 J.
C)Both the system and the surroundings are gaining 95 J.
D)Both the system and the surroundings are losing 95 J.
E)None of the above are true.
The system is losing 95 J,while the surroundings are gaining 95 J.
2
Which of the following (with specific heat capacity provided)would show the smallest temperature change upon gaining 200.0 J of heat?
A)50.0 g Al,CAl = 0.903 J/g°C
B)50.0 g Cu,CCu = 0.385 J/g°C
C)25.0 g granite,Cgranite = 0.79 J/g°C
D)25.0 g Au,CAu = 0.128 J/g°C
E)25.0 g Ag,CAg = 0.235 J/g°C
A)50.0 g Al,CAl = 0.903 J/g°C
B)50.0 g Cu,CCu = 0.385 J/g°C
C)25.0 g granite,Cgranite = 0.79 J/g°C
D)25.0 g Au,CAu = 0.128 J/g°C
E)25.0 g Ag,CAg = 0.235 J/g°C
50.0 g Al,CAl = 0.903 J/g°C
3
Calculate the amount of heat (in kJ)necessary to raise the temperature of 47.8 g benzene by 57.0 K.The specific heat capacity of benzene is 1.05 J/g°C
A)1.61 kJ
B)16.6 kJ
C)2.59 kJ
D)2.86 kJ
E)3.85 kJ
A)1.61 kJ
B)16.6 kJ
C)2.59 kJ
D)2.86 kJ
E)3.85 kJ
2.86 kJ
4
Calculate the amount of heat (in kJ)required to raise the temperature of a 79.0 g sample of ethanol from 298.0 K to 385.0 K.The specific heat capacity of ethanol is 2.42 J/g°C.
A)57.0 kJ
B)16.6 kJ
C)73.6 kJ
D)28.4 kJ
E)12.9 kJ
A)57.0 kJ
B)16.6 kJ
C)73.6 kJ
D)28.4 kJ
E)12.9 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following signs on q and w represent a system that is doing work on the surroundings,as well as gaining heat from the surroundings?
A)q = +,w = -
B)q = -,w = +
C)q = +,w = +
D)q = -,w = -
E)None of these represent the system referenced above.
A)q = +,w = -
B)q = -,w = +
C)q = +,w = +
D)q = -,w = -
E)None of these represent the system referenced above.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
A 4.98 g sample of aniline (C6H5NH2,molar mass = 93.13 g/mol)was combusted in a bomb calorimeter with a heat capacity of 4.25 kJ/°C.If the temperature rose from 29.5°C to 69.8°C,determine the value of ΔH°comb for aniline.
A)+7.81 × 103 kJ/mol
B)-3.20 × 103 kJ/mol
C)+1.71 × 103 kJ/mol
D)-1.71 × 103 kJ/mol
E)-7.81 × 103 kJ/mol
A)+7.81 × 103 kJ/mol
B)-3.20 × 103 kJ/mol
C)+1.71 × 103 kJ/mol
D)-1.71 × 103 kJ/mol
E)-7.81 × 103 kJ/mol

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
Determine the specific heat capacity of an alloy that requires 59.3 kJ to raise the temperature of 150.0 g alloy from 298 K to 398 K.
A)4.38 J/g°C
B)2.29 J/g°C
C)3.95 J/g°C
D)2.53 J/g°C
E)1.87 J/g°C
A)4.38 J/g°C
B)2.29 J/g°C
C)3.95 J/g°C
D)2.53 J/g°C
E)1.87 J/g°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
A 21.8 g sample of ethanol (C2H5OH)is burned in a bomb calorimeter,according to the following reaction.If the temperature rises from 25.0 to 62.3°C,determine the heat capacity of the calorimeter.The molar mass of ethanol is 46.07 g/mol.
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = -1235 kJ
A)4.99 kJ/°C
B)5.65 kJ/°C
C)63.7 kJ/°C
D)33.1 kJ/°C
E)15.7 kJ/°C
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = -1235 kJ
A)4.99 kJ/°C
B)5.65 kJ/°C
C)63.7 kJ/°C
D)33.1 kJ/°C
E)15.7 kJ/°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following signs on q and w represent a system that is doing work on the surroundings,as well as losing heat to the surroundings?
A)q = - ,w = -
B)q = +,w = +
C)q = -,w = +
D)q = +,w = -
E)None of these represent the system referenced above.
A)q = - ,w = -
B)q = +,w = +
C)q = -,w = +
D)q = +,w = -
E)None of these represent the system referenced above.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
A piece of iron (C = 0.449 J/g°C)and a piece of gold (C = 0.128 J/g°C)have identical masses.If the iron has an initial temperature of 498 K and the gold has an initial temperature of 298 K,which of the following statements is TRUE of the outcome when the two metals are placed in contact with one another? Assume no heat is lost to the surroundings.
A)Since the two metals have the same mass,the final temperature of the two metals will be 398 K,exactly half way in between the two initial temperatures.
B)Since the two metals have the same mass,but the specific heat capacity of gold is much smaller than that of iron,the final temperature of the two metals will be closer to 298 K than to 498 K.
C)Since the two metals have the same mass,the thermal energy contained in the iron and gold after reaching thermal equilibrium will be the same.
D)Since the two metals have the same mass,but the specific heat capacity of iron is much greater than that of gold,the final temperature of the two metals will be closer to 498 K than to 298 K.
E)None of the above are true.
A)Since the two metals have the same mass,the final temperature of the two metals will be 398 K,exactly half way in between the two initial temperatures.
B)Since the two metals have the same mass,but the specific heat capacity of gold is much smaller than that of iron,the final temperature of the two metals will be closer to 298 K than to 498 K.
C)Since the two metals have the same mass,the thermal energy contained in the iron and gold after reaching thermal equilibrium will be the same.
D)Since the two metals have the same mass,but the specific heat capacity of iron is much greater than that of gold,the final temperature of the two metals will be closer to 498 K than to 298 K.
E)None of the above are true.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the change internal energy (ΔE)for a system that is giving off 45.0 kJ of heat and is performing 855 J of work on the surroundings.
A)44.1 kJ
B)-44.1 kJ
C)-45.9 kJ
D)9.00 × 102 kJ
E)-9.00 × 102 kJ
A)44.1 kJ
B)-44.1 kJ
C)-45.9 kJ
D)9.00 × 102 kJ
E)-9.00 × 102 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
A sample of copper absorbs 43.6 kJ of heat,resulting in a temperature rise of 75.0°C,determine the mass (in kg)of the copper sample if the specific heat capacity of copper is 0.385 J/g°C.
A)1.51 kg
B)6.62 kg
C)1.26 kg
D)7.94 kg
E)3.64 kg
A)1.51 kg
B)6.62 kg
C)1.26 kg
D)7.94 kg
E)3.64 kg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
For ?Esys to always be -,what must be true?
A)q = w
B)+q > -w
C)+w > -q
D)-w > +q
A)q = w
B)+q > -w
C)+w > -q
D)-w > +q

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
A 6.55 g sample of aniline (C6H5NH2,molar mass = 93.13 g/mol)was combusted in a bomb calorimeter.If the temperature rose by 32.9°C,use the information below to determine the heat capacity of the calorimeter.
4 C6H5NH2(l)+ 35 O2(g)→ 24 CO2(g)+ 14 H2O(g)+ 4 NO2(g)
ΔH°rxn= -1.28 × 104 kJ
A)97.3 kJ/°C
B)38.9 kJ/°C
C)5.94 kJ/°C
D)6.84 kJ/°C
E)12.8 kJ/°C
4 C6H5NH2(l)+ 35 O2(g)→ 24 CO2(g)+ 14 H2O(g)+ 4 NO2(g)
ΔH°rxn= -1.28 × 104 kJ
A)97.3 kJ/°C
B)38.9 kJ/°C
C)5.94 kJ/°C
D)6.84 kJ/°C
E)12.8 kJ/°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the change in internal energy (ΔE)for a system that is giving off 25.0 kJ of heat and is changing from 12.00 L to 6.00 L in volume at 1.50 atm pressure.(Remember that 101.3 J = 1 L∙atm)
A)+25.9 kJ
B)-16.0 kJ
C)-25.9 kJ
D)-24.1 kJ
E)937 kJ
A)+25.9 kJ
B)-16.0 kJ
C)-25.9 kJ
D)-24.1 kJ
E)937 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following substances (with specific heat capacity provided)would show the greatest temperature change upon absorbing 100.0 J of heat?
A)10.0 g Ag,CAg = 0.235 J/g°C
B)10.0 g H2O,CH2O = 4.18 J/g°C
C)10.0 g ethanol,Cethanol = 2.42 J/g°C
D)10.0 g Fe,CFe = 0.449 J/g°C
E)10.0 g Au,CAu = 0.128 J/g°C
A)10.0 g Ag,CAg = 0.235 J/g°C
B)10.0 g H2O,CH2O = 4.18 J/g°C
C)10.0 g ethanol,Cethanol = 2.42 J/g°C
D)10.0 g Fe,CFe = 0.449 J/g°C
E)10.0 g Au,CAu = 0.128 J/g°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
Energy that is associated with the position or composition of an object is called
A)kinetic energy.
B)thermal energy.
C)potential energy.
D)chemical energy.
A)kinetic energy.
B)thermal energy.
C)potential energy.
D)chemical energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
Calculate the change in internal energy (ΔE)for a system that is absorbing 35.8 kJ of heat and is expanding from 8.00 to 24.0 L in volume at 1.00 atm.(Remember that 101.3 J = 1 L∙atm)
A)+51.8 kJ
B)-15.8 kJ
C)-16.6 kJ
D)-29.3 kJ
E)+34.2 kJ
A)+51.8 kJ
B)-15.8 kJ
C)-16.6 kJ
D)-29.3 kJ
E)+34.2 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Determine the final temperature of a gold nugget (mass = 376 g)that starts at 398 K and loses 4.85 kJ of heat to a snowbank when it is lost.The specific heat capacity of gold is 0.128 J/g°C.
A)133 K
B)398 K
C)187 K
D)297 K
E)377 K
A)133 K
B)398 K
C)187 K
D)297 K
E)377 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
A sample of 5.23 kg of aluminum absorbs heat,which raises the temperature 50.0°C.Determine the amount of heat absorbed,in kJ,if the specific heat capacity of aluminum is 0.903 J/g°C.
A)0.0405 kJ
B)290 kJ
C)236 kJ
D)8.60 kJ
A)0.0405 kJ
B)290 kJ
C)236 kJ
D)8.60 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
According to the following reaction,how much energy is required to decompose 55.0 kg of Fe3O4? The molar mass of Fe3O4 is 231.55 g/mol.
Fe3O4(s)→ 3 Fe(s)+ 2 O2(g)ΔH°rxn = +1118 kJ
A)1.10 × 106 kJ
B)2.38 × 102 kJ
C)2.66 × 105 kJ
D)1.12 × 103 kJ
E)3.44 × 104 kJ
Fe3O4(s)→ 3 Fe(s)+ 2 O2(g)ΔH°rxn = +1118 kJ
A)1.10 × 106 kJ
B)2.38 × 102 kJ
C)2.66 × 105 kJ
D)1.12 × 103 kJ
E)3.44 × 104 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
Using the following thermochemical equation,determine the amount of heat produced per kg of CO2 formed during the combustion of benzene (C6H6).
2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A)1.43 × 105 kJ/kg CO2
B)2.30 × 104 kJ/kg CO2
C)4.34 × 104 kJ/kg CO2
D)1.19 × 104 kJ/kg CO2
E)8.40 × 105 kJ/kg CO2
2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A)1.43 × 105 kJ/kg CO2
B)2.30 × 104 kJ/kg CO2
C)4.34 × 104 kJ/kg CO2
D)1.19 × 104 kJ/kg CO2
E)8.40 × 105 kJ/kg CO2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
How much energy is evolved during the reaction of 48.7 g of Al,according to the reaction below? Assume that there is excess Fe2O3.
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A)415 kJ
B)207 kJ
C)241 kJ
D)130 kJ
E)769 kJ
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A)415 kJ
B)207 kJ
C)241 kJ
D)130 kJ
E)769 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
A 35.6 g sample of ethanol (C2H5OH)is burned in a bomb calorimeter,according to the following reaction.If the temperature rose from 35.0 to 76.0°C and the heat capacity of the calorimeter is 23.3 kJ/°C,what is the value of ΔH°rxn? The molar mass of ethanol is 46.07 g/mol.
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = ?
A)-1.24 × 103 kJ/mol
B)+1.24 × 103 kJ/mol
C)-8.09 × 103 kJ/mol
D)-9.55 × 103 kJ/mol
E)+9.55 × 103 kJ/mol
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = ?
A)-1.24 × 103 kJ/mol
B)+1.24 × 103 kJ/mol
C)-8.09 × 103 kJ/mol
D)-9.55 × 103 kJ/mol
E)+9.55 × 103 kJ/mol

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
The temperature rises from 25.00 °C to 29.00 °C in a bomb calorimeter when 3.50 g of sucrose undergoes combustion in a bomb calorimeter.Calculate ΔErxn for the combustion of sucrose in kJ/mol sucrose.The heat capacity of the calorimeter is 4.90 kJ/°C .The molar mass of sugar is 342.3 g/mol.
A)()- 1.92 × 103 kJ/mole
B)1.92 × 103 kJ/mole
C)()- 1.23 × 103 kJ/mole
D)2.35 × 104 kJ/mole
A)()- 1.92 × 103 kJ/mole
B)1.92 × 103 kJ/mole
C)()- 1.23 × 103 kJ/mole
D)2.35 × 104 kJ/mole

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
According to the following thermochemical equation,what mass of H2O (in g)must form in order to produce 975 kJ of energy?
SiO2(s)+ 4 HF(g)→ SiF4(g)+ 2 H2O(l)ΔH°rxn = -184 kJ
A)68.0 g
B)102 g
C)54.1 g
D)191 g
E)95.5 g
SiO2(s)+ 4 HF(g)→ SiF4(g)+ 2 H2O(l)ΔH°rxn = -184 kJ
A)68.0 g
B)102 g
C)54.1 g
D)191 g
E)95.5 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
What volume of benzene (C6H6,d= 0.88 g/mL,molar mass = 78.11 g/mol)is required to produce 1.5 x 103 kJ of heat according to the following reaction?
2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A)75 mL
B)37 mL
C)21 mL
D)19 mL
E)42 mL
2 C6H6(l)+ 15 O2(g)→ 12 CO2(g)+ 6 H2O(g)ΔH°rxn = -6278 kJ
A)75 mL
B)37 mL
C)21 mL
D)19 mL
E)42 mL

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
According to the following reaction,how much energy is evolved during the reaction of 2.50 L B2H6 and 5.65 L Cl2 (Both gases are initially at STP)? The molar mass of B2H6 is 27.67 g/mol.
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A)58.7 kJ
B)156 kJ
C)215 kJ
D)352 kJ
E)508 kJ
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A)58.7 kJ
B)156 kJ
C)215 kJ
D)352 kJ
E)508 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
What is the minimum energy required to decompose 765 g of PCl3,according to the reaction below? The molar mass of PCl3 is 137.32 g/mol and may be useful.
4 PCl3(g)→ P4(s)+ 6 Cl2(g)ΔH°rxn = +1207 kJ
A)2.31 × 103 kJ
B)4.33 × 103 kJ
C)6.72 × 103 kJ
D)1.68 × 103 kJ
E)5.95 × 103 kJ
4 PCl3(g)→ P4(s)+ 6 Cl2(g)ΔH°rxn = +1207 kJ
A)2.31 × 103 kJ
B)4.33 × 103 kJ
C)6.72 × 103 kJ
D)1.68 × 103 kJ
E)5.95 × 103 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
Using the following equation for the combustion of octane,calculate the heat of reaction for 100.0 g of octane.The molar mass of octane is 114.33 g/mole.
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH°rxn = -11018 kJ
A)-4.82 x 103 kJ
B)-4.82 kJ
C)-9.64 × 103 kJ
D)-1.26 × 104 kJ
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH°rxn = -11018 kJ
A)-4.82 x 103 kJ
B)-4.82 kJ
C)-9.64 × 103 kJ
D)-1.26 × 104 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following processes is endothermic?
A)the freezing of water
B)the combustion of propane
C)a hot cup of coffee (system)cooling on a countertop
D)the chemical reaction in a "hot pack" often used to treat sore muscles
E)the vaporization of rubbing alcohol
A)the freezing of water
B)the combustion of propane
C)a hot cup of coffee (system)cooling on a countertop
D)the chemical reaction in a "hot pack" often used to treat sore muscles
E)the vaporization of rubbing alcohol

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following processes is endothermic?
A)An atom emits a photon.
B)the condensation of water
C)An atom absorbs a photon.
D)the electron affinity of a fluorine atom
E)None of the above processes are endothermic.
A)An atom emits a photon.
B)the condensation of water
C)An atom absorbs a photon.
D)the electron affinity of a fluorine atom
E)None of the above processes are endothermic.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
Given w = 0,an endothermic reaction has the following.
A)+ΔH and -ΔE
B)-ΔH and +ΔE
C)+ΔH and +ΔE
D)-ΔH and -ΔE
A)+ΔH and -ΔE
B)-ΔH and +ΔE
C)+ΔH and +ΔE
D)-ΔH and -ΔE

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
According to the following thermochemical equation,what mass of HF (in g)must react in order to produce 345 kJ of energy? Assume excess SiO2.
SiO2(s)+ 4 HF(g)→ SiF4(g)+ 2 H2O(l)ΔH°rxn = -184 kJ
A)42.7 g
B)37.5 g
C)150.g
D)107 g
E)173 g
SiO2(s)+ 4 HF(g)→ SiF4(g)+ 2 H2O(l)ΔH°rxn = -184 kJ
A)42.7 g
B)37.5 g
C)150.g
D)107 g
E)173 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
A 12.8 g sample of ethanol (C2H5OH)is burned in a bomb calorimeter with a heat capacity of 5.65 kJ/°C.Using the information below,determine the final temperature of the calorimeter if the initial temperature is 25.0°C.The molar mass of ethanol is 46.07 g/mol.
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = -1235 kJ
A)53.4°C
B)28.1°C
C)111°C
D)85.7°C
E)74.2°C
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(g)ΔH°rxn = -1235 kJ
A)53.4°C
B)28.1°C
C)111°C
D)85.7°C
E)74.2°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
A 6.55 g sample of aniline (C6H5NH2,molar mass = 93.13 g/mol)was combusted in a bomb calorimeter with a heat capacity of 14.25 kJ/°C.If the initial temperature was 32.9°C,use the information below to determine the value of the final temperature of the calorimeter.
4 C6H5NH2(l)+ 35 O2(g)→ 24 CO2(g)+ 14 H2O(g)+ 4 NO2(g)
ΔH°rxn = -1.28 × 104 kJ
A)257°C
B)46.6°C
C)48.7°C
D)41.9°C
E)931°C
4 C6H5NH2(l)+ 35 O2(g)→ 24 CO2(g)+ 14 H2O(g)+ 4 NO2(g)
ΔH°rxn = -1.28 × 104 kJ
A)257°C
B)46.6°C
C)48.7°C
D)41.9°C
E)931°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
An exothermic reaction has
A)a negative ΔH and absorbs heat from the surroundings.An exothermic reaction feels warm to the touch.
B)a positive ΔH and absorbs heat from the surroundings.An exothermic reaction feels warm to the touch.
C)a positive ΔH and gives off heat to the surroundings.An exothermic reaction feels warm to the touch.
D)a negative ΔH and gives off heat to the surroundings.An exothermic reaction feels warm to the touch.
A)a negative ΔH and absorbs heat from the surroundings.An exothermic reaction feels warm to the touch.
B)a positive ΔH and absorbs heat from the surroundings.An exothermic reaction feels warm to the touch.
C)a positive ΔH and gives off heat to the surroundings.An exothermic reaction feels warm to the touch.
D)a negative ΔH and gives off heat to the surroundings.An exothermic reaction feels warm to the touch.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following processes is exothermic?
A)the formation of dew in the morning
B)the melting of ice
C)the chemical reaction in a "cold pack" often used to treat injuries
D)the vaporization of water
E)None of the above are exothermic.
A)the formation of dew in the morning
B)the melting of ice
C)the chemical reaction in a "cold pack" often used to treat injuries
D)the vaporization of water
E)None of the above are exothermic.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
How much energy is evolved during the formation of 98.7 g of Fe,according to the reaction below?
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A)753 kJ
B)1.51 × 103 kJ
C)4.20 × 103 kJ
D)482 kJ
E)241 kJ
Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)ΔH°rxn = -852 kJ
A)753 kJ
B)1.51 × 103 kJ
C)4.20 × 103 kJ
D)482 kJ
E)241 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
According to the following reaction,how much energy is evolved during the reaction of 32.5 g B2H6 and 72.5 g Cl2? The molar mass of B2H6 is 27.67 g/mol.
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A)1640 kJ
B)238 kJ
C)1430 kJ
D)3070 kJ
E)429 kJ
B2H6(g)+ 6 Cl2(g)→ 2 BCl3(g)+ 6 HCl(g)ΔH°rxn = -1396 kJ
A)1640 kJ
B)238 kJ
C)1430 kJ
D)3070 kJ
E)429 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction:
P4(g)+ 10 Cl2(g)→ 4PCl5(s)ΔH°rxn = ?
Given:
PCl5(s)→ PCl3(g)+ Cl2(g)ΔH°rxn= +157 kJ
P4(g)+ 6 Cl2(g)→ 4 PCl3(g)ΔH°rxn = -1207 kJ
A)-1835 kJ
B)-1364 kJ
C)-1050.kJ
D)-1786 kJ
E)-2100.kJ
P4(g)+ 10 Cl2(g)→ 4PCl5(s)ΔH°rxn = ?
Given:
PCl5(s)→ PCl3(g)+ Cl2(g)ΔH°rxn= +157 kJ
P4(g)+ 6 Cl2(g)→ 4 PCl3(g)ΔH°rxn = -1207 kJ
A)-1835 kJ
B)-1364 kJ
C)-1050.kJ
D)-1786 kJ
E)-2100.kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements is true?
A)State functions do not depend on the path taken to arrive at a particular state.
B)DErxn can be determined using constant volume calorimetry.
C)Energy is neither created nor destroyed,excluding nuclear reactions.
D)ΔHrxn can be determined using constant pressure calorimetry.
E)All of the above are true.
A)State functions do not depend on the path taken to arrive at a particular state.
B)DErxn can be determined using constant volume calorimetry.
C)Energy is neither created nor destroyed,excluding nuclear reactions.
D)ΔHrxn can be determined using constant pressure calorimetry.
E)All of the above are true.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
Use the ΔH°f information provided to calculate ΔH°rxn for the following:
ΔH°f (kJ/mol) SO2Cl2(g)+ 2 H2O(l)→ 2 HCl(g)+ H2SO4(l)ΔH°rxn = ?
SO2Cl2(g)-364
H2O(l)-286
HCl(g)-92
H2SO4(l)-814
A)-256 kJ
B)+161 kJ
C)-62 kJ
D)+800.kJ
E)-422 kJ
ΔH°f (kJ/mol) SO2Cl2(g)+ 2 H2O(l)→ 2 HCl(g)+ H2SO4(l)ΔH°rxn = ?
SO2Cl2(g)-364
H2O(l)-286
HCl(g)-92
H2SO4(l)-814
A)-256 kJ
B)+161 kJ
C)-62 kJ
D)+800.kJ
E)-422 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
A piece of iron (mass = 25.0 g)at 398 K is placed in a styrofoam coffee cup containing 25.0 mL of water at 298 K.Assuming that no heat is lost to the cup or the surroundings,what will the final temperature of the water be? The specific heat capacity of iron = 0.449 J/g°C and water = 4.18 J/g°C.
A)348 K
B)308 K
C)287 K
D)325 K
E)388 K
A)348 K
B)308 K
C)287 K
D)325 K
E)388 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
Use the information provided to determine ΔH°rxn for the following reaction:
ΔH°f (kJ/mol)3 Fe2O3(s)+ CO(g)→ 2 Fe3O4(s)+ CO2(g)ΔH°rxn = ? kJ
Fe2O3(s)-824
Fe3O4(s)-1118
CO(g)-111
CO2(g)-394
A)+277 kJ
B)-577 kJ
C)-47 kJ
D)+144 kJ
E)-111 kJ
ΔH°f (kJ/mol)3 Fe2O3(s)+ CO(g)→ 2 Fe3O4(s)+ CO2(g)ΔH°rxn = ? kJ
Fe2O3(s)-824
Fe3O4(s)-1118
CO(g)-111
CO2(g)-394
A)+277 kJ
B)-577 kJ
C)-47 kJ
D)+144 kJ
E)-111 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
A 100.0 mL sample of 0.300 M NaOH is mixed with a 100.0 mL sample of 0.300 M HNO3 in a coffee cup calorimeter.If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C,determine the ΔH°rxn (in units of kJ/mol NaOH)for the neutralization reaction between aqueous NaOH and HCl.Assume 1)that no heat is lost to the calorimeter or the surroundings,and 2)that the density and the heat capacity of the resulting solution are the same as water.
A)-55.7 kJ/mol NaOH
B)-169 kJ/mol NaOH
C)-16.7 kJ/mol NaOH
D)-27.9 kJ/mol NaOH
E)- 34.4 kJ/mol NaOH
A)-55.7 kJ/mol NaOH
B)-169 kJ/mol NaOH
C)-16.7 kJ/mol NaOH
D)-27.9 kJ/mol NaOH
E)- 34.4 kJ/mol NaOH

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
Use the information provided to determine ΔH°rxn for the following reaction:
ΔH°f (kJ/mol)CH4(g)+ 3 Cl2(g)→ CHCl3(l)+ 3 HCl(g)ΔH°rxn = ?
CH4(g)-75
CHCl3(l)-134
HCl(g)-92
A)-151 kJ
B)-335 kJ
C)+662 kJ
D)+117 kJ
E)-217 kJ
ΔH°f (kJ/mol)CH4(g)+ 3 Cl2(g)→ CHCl3(l)+ 3 HCl(g)ΔH°rxn = ?
CH4(g)-75
CHCl3(l)-134
HCl(g)-92
A)-151 kJ
B)-335 kJ
C)+662 kJ
D)+117 kJ
E)-217 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
Use the information provided to determine ΔH°rxn for the following reaction:
ΔH°f (kJ/mol)CH4(g)+ 4 Cl2(g)→ CCl4(g)+ 4 HCl(g)ΔH°rxn = ?
CH4(g)-75
CCl4(g)-96
HCl(g)-92
A)-389 kJ
B)-113 kJ
C)+113 kJ
D)-71 kJ
E)+79 kJ
ΔH°f (kJ/mol)CH4(g)+ 4 Cl2(g)→ CCl4(g)+ 4 HCl(g)ΔH°rxn = ?
CH4(g)-75
CCl4(g)-96
HCl(g)-92
A)-389 kJ
B)-113 kJ
C)+113 kJ
D)-71 kJ
E)+79 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction:
2 S(s)+ 3 O2(g)→ 2SO3(g)ΔH°rxn = ?
Given:
SO2(g)→ S(s)+ O2(g)ΔH°rxn = +296.8 kJ
2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -197.8 kJ
A)-494.6 kJ
B)-692.4 kJ
C)-791.4 kJ
D)-98.8 kJ
E)-293.0 kJ
2 S(s)+ 3 O2(g)→ 2SO3(g)ΔH°rxn = ?
Given:
SO2(g)→ S(s)+ O2(g)ΔH°rxn = +296.8 kJ
2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -197.8 kJ
A)-494.6 kJ
B)-692.4 kJ
C)-791.4 kJ
D)-98.8 kJ
E)-293.0 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements are false?
A)If a chemical equation is multiplied by some factor,ΔH°rxn is also multiplied by the same factor.
B)If a chemical reaction changes direction,then ΔH°rxn changes sign.
C)If a chemical equation can be expressed as the sum of a series of steps,ΔH°rxn for the overall equation is the sum of the heats of reactions for each step.
D)Standard states are all in the gas phase.
A)If a chemical equation is multiplied by some factor,ΔH°rxn is also multiplied by the same factor.
B)If a chemical reaction changes direction,then ΔH°rxn changes sign.
C)If a chemical equation can be expressed as the sum of a series of steps,ΔH°rxn for the overall equation is the sum of the heats of reactions for each step.
D)Standard states are all in the gas phase.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
Match the following.
+w
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings
+w
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
Use the ΔH°f and ΔH°rxn information provided to calculate ΔH°f for IF:
ΔH°f (kJ/mol) IF7(g)+ I2(g)→ IF5(g)+ 2 IF(g)ΔH°rxn = -89 kJ
IF7(g)-941
IF5(g)-840
A)101 kJ/mol
B)-146 kJ/mol
C)-190.kJ/mol
D)-95 kJ/mol
E)24 kJ/mol
ΔH°f (kJ/mol) IF7(g)+ I2(g)→ IF5(g)+ 2 IF(g)ΔH°rxn = -89 kJ
IF7(g)-941
IF5(g)-840
A)101 kJ/mol
B)-146 kJ/mol
C)-190.kJ/mol
D)-95 kJ/mol
E)24 kJ/mol

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
Match the following.
-w
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings
-w
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following processes is exothermic?
A)the ionization of a lithium atom
B)the breaking of a Cl-Cl bond
C)the sublimation of dry ice (CO2(s))
D)the reaction associated with ΔH∘f for an ionic compound
E)All of the above processes are exothermic.
A)the ionization of a lithium atom
B)the breaking of a Cl-Cl bond
C)the sublimation of dry ice (CO2(s))
D)the reaction associated with ΔH∘f for an ionic compound
E)All of the above processes are exothermic.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
Choose the reaction that illustrates ΔH°f for Ca(NO3)2.
A)Ca(s)+ N2(g)+ 3O2(g)→ Ca(NO3)2(s)
B)Ca2+(aq)+ 2 NO3-(aq)→ Ca(NO3)2(aq)
C)Ca(s)+ 2 N(g)+ 6 O(g)→ Ca(NO3)2(s)
D)Ca(NO3)2(aq)→ Ca2+(aq)+ 2 NO3-(aq)
E)Ca(NO3)2(s)→ Ca(s)+ N2(g)+ 3O2(g)
A)Ca(s)+ N2(g)+ 3O2(g)→ Ca(NO3)2(s)
B)Ca2+(aq)+ 2 NO3-(aq)→ Ca(NO3)2(aq)
C)Ca(s)+ 2 N(g)+ 6 O(g)→ Ca(NO3)2(s)
D)Ca(NO3)2(aq)→ Ca2+(aq)+ 2 NO3-(aq)
E)Ca(NO3)2(s)→ Ca(s)+ N2(g)+ 3O2(g)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter.The reaction causes the temperature of the resulting solution to fall below room temperature.Which of the following statements is true?
A)The products have a lower potential energy than the reactants.
B)This type of experiment will provide data to calculate ΔErxn.
C)The reaction is exothermic.
D)Energy is leaving the system during reaction.
E)None of the above statements are true.
A)The products have a lower potential energy than the reactants.
B)This type of experiment will provide data to calculate ΔErxn.
C)The reaction is exothermic.
D)Energy is leaving the system during reaction.
E)None of the above statements are true.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
Use the standard reaction enthalpies given below to determine ΔH°rxn for the following reaction:
2 NO(g)+ O2(g)→ 2 NO2(g)ΔH°rxn = ?
Given:
N2(g)+ O2(g)→ 2 NO(g)ΔH°rxn = +183 kJ
1/2 N2(g)+ O2(g)→ NO2(g)ΔH°rxn = +33 kJ
A)-150.kJ
B)-117 kJ
C)-333 kJ
D)+115 kJ
E)+238 kJ
2 NO(g)+ O2(g)→ 2 NO2(g)ΔH°rxn = ?
Given:
N2(g)+ O2(g)→ 2 NO(g)ΔH°rxn = +183 kJ
1/2 N2(g)+ O2(g)→ NO2(g)ΔH°rxn = +33 kJ
A)-150.kJ
B)-117 kJ
C)-333 kJ
D)+115 kJ
E)+238 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
Use the ΔH°f and ΔH°rxn information provided to calculate ΔH°f for SO3(g):
ΔH°f (kJ/mol) 2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -198 kJ
SO2(g)-297
A)-792 kJ/mol
B)-248 kJ/mol
C)-495 kJ/mol
D)-578 kJ/mol
E)-396 kJ/mol
ΔH°f (kJ/mol) 2 SO2(g)+ O2(g)→ 2 SO3(g)ΔH°rxn = -198 kJ
SO2(g)-297
A)-792 kJ/mol
B)-248 kJ/mol
C)-495 kJ/mol
D)-578 kJ/mol
E)-396 kJ/mol

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
Choose the thermochemical equation that illustrates ΔH°f for Li2SO4.
A)2 Li+(aq)+ SO42-(aq)→ Li2SO4(aq)
B)2 Li(s)+ 1/8 S8(s,rhombic)+ 2 O2(g)→ Li2SO4(s)
C)Li2SO4(aq)→ 2 Li+(aq)+ SO42-(aq)
D)8 Li2SO4(s)→ 16 Li(s)+ S8(s,rhombic)+ 16 O2(g)
E)16 Li(s)+ S8(s,rhombic)+ 16 O2(g)→ 8 Li2SO4(s)
A)2 Li+(aq)+ SO42-(aq)→ Li2SO4(aq)
B)2 Li(s)+ 1/8 S8(s,rhombic)+ 2 O2(g)→ Li2SO4(s)
C)Li2SO4(aq)→ 2 Li+(aq)+ SO42-(aq)
D)8 Li2SO4(s)→ 16 Li(s)+ S8(s,rhombic)+ 16 O2(g)
E)16 Li(s)+ S8(s,rhombic)+ 16 O2(g)→ 8 Li2SO4(s)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
Match the following.
-ΔE
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings
-ΔE
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
Explain the difference between ΔH and ΔE.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
A 6.50-g sample of copper metal at 25.0°C is heated by the addition of 84.0 J of energy.The final temperature of the copper is ________°C.The specific heat capacity of copper is 0.38 J/g ∙ K.
A)29.9
B)25.0
C)9.0
D)59.0
E)34.0
A)29.9
B)25.0
C)9.0
D)59.0
E)34.0

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
The specific heat capacity of liquid water is 4.18 J/g ∙ K.How many joules of heat are needed to raise the temperature of 5.00 g of water from 25.1°C to 65.3°C?
A)48.1 J
B)840 J
C)1.89 ×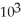
J
D)2.08 ×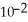
J
E)54.4 J
A)48.1 J
B)840 J
C)1.89 ×
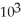
J
D)2.08 ×
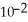
J
E)54.4 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the kinetic energy of a 150 g baseball moving at a speed of 39.m/s (87 mph).
A)5.8 J
B)1.1 × 102 J
C)5.8 × 103 J
D)1.1 × 105 J
A)5.8 J
B)1.1 × 102 J
C)5.8 × 103 J
D)1.1 × 105 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
Give the equation to calculate the enthalpy of a system.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
A 5.00-g sample of liquid water at 25.0 C is heated by the addition of 84.0 J of energy.The final temperature of the water is ________ °C.The specific heat capacity of liquid water is 4.18 J/g ∙ K.
A)95.2
B)25.2
C)-21.0
D)29.0
E)4.02
A)95.2
B)25.2
C)-21.0
D)29.0
E)4.02

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
For a process at constant pressure,49,600 calories of heat are released.This quantity of heat is equivalent to
A)4.82 × 10-6 J.
B)1.19 × 104 J.
C)1.24 × 104 J.
D)2.08 × 105 J.
A)4.82 × 10-6 J.
B)1.19 × 104 J.
C)1.24 × 104 J.
D)2.08 × 105 J.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the work,w,gained or lost by the system when a gas expands from 15 L to 40 L against a constant external pressure of 1.5 atm.1 L ∙ atm = 101 J.
A)-6.1 kJ
B)-3.8 kJ
C)+3.8 kJ
D)+6.1 kJ
A)-6.1 kJ
B)-3.8 kJ
C)+3.8 kJ
D)+6.1 kJ

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
Match the following.
+q
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings
+q
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
Match the following.
-q
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings
-q
A)work done by the system on the surroundings
B)system gains thermal energy from surroundings
C)energy flows out of system into the surroundings
D)system loses thermal energy to surroundings
E)work done on the system by the surroundings
F)energy flows into the system from the surroundings

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
The specific heat capacity of liquid mercury is 0.14 J/g ∙ K.How many joules of heat are needed to raise the temperature of 5.00 g of mercury from 15.0°C to 36.5°C?
A)7.7 × 102 J
B)15 J
C)36 J
D)0.0013 J
E)1.7 J
A)7.7 × 102 J
B)15 J
C)36 J
D)0.0013 J
E)1.7 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
Why are the standard heats of formation for elements in their most stable form assigned a value of "0"?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
Describe the energy changes that occur when a book is held 6 ft off the floor and then dropped.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
Define chemical energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
Give the temperature and pressure for the standard state for a liquid.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
It takes 11.2 kJ of energy to raise the temperature of 145 g of benzene from 23.0°C to 68.0°C.What is the specific heat of benzene?
A)1.14 J/(g ∙ °C)
B)1.72 J/(g ∙ °C)
C)3.48 J/(g ∙ °C)
D)5.25 J/(g ∙ °C)
A)1.14 J/(g ∙ °C)
B)1.72 J/(g ∙ °C)
C)3.48 J/(g ∙ °C)
D)5.25 J/(g ∙ °C)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
The specific heat of copper is 0.385 J/(g ∙ °C).If 34.2 g of copper,initially at 24.0°C,absorbs 4.689 kJ,what will be the final temperature of the copper?
A)24.4°C
B)26.8°C
C)356°C
D)380°C
A)24.4°C
B)26.8°C
C)356°C
D)380°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
Where does the energy absorbed during an endothermic reaction go?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
The specific heat capacity of methane gas is 2.20 J/g ∙ K.How many joules of heat are needed to raise the temperature of 5.00 g of methane from 36.0°C to 75.0°C?
A)88.6 J
B)429 J
C)1221 J
D)0.0113 J
E)22.9 J
A)88.6 J
B)429 J
C)1221 J
D)0.0113 J
E)22.9 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
The specific heat capacity of solid copper metal is 0.385 J/g ∙ K.How many joules of heat are needed to raise the temperature of a 1.55-kg block of copper from 33.0°C to 77.5°C?
A)1.79 × 105 J
B)26.6 J
C)2.66 × 104 J
D)5.58 × 10-6 J
E)0.00558 J
A)1.79 × 105 J
B)26.6 J
C)2.66 × 104 J
D)5.58 × 10-6 J
E)0.00558 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


